Preparation method of CaCu3Ti4O12
A powder and crystal technology, applied in the field of electronic ceramics preparation and application, can solve the problems of unfavorable CCTO crystal dynamic characteristics, cumbersome preparation process, and obtaining CCTO crystal powder, and achieve narrow particle size distribution range, simple process, and sintering process Optimized effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation method of the present embodiment CCTO crystal powder is based on calcium acetylacetonate Ca (acac) 2 , Copper acetylacetonate Cu(acac) 2 and titanyl acetylacetonate TiO(acac) 2 As a raw material, using absolute ethanol as a solvent and anhydrous citric acid as a stabilizer, the CCTO crystal powder is obtained by low-temperature calcination, which specifically includes the following steps:
[0033] (1) Weigh 0.01mol of Ca(acac) 2 , 0.03mol of Cu(acac) 2 and 0.04mol of TiO(acac) 2 , add 20ml of absolute ethanol, and stir vigorously with a magnetic stirrer to obtain a mixed solution;
[0034] (2) Add anhydrous citric acid to the mixed solution to adjust the pH value to 1.5 to obtain a light blue solution, and continue to stir for 1 hour;
[0035] (3) drying the solution in step (2) at 80° C. for 12 hours to obtain light blue needle-like crystals;
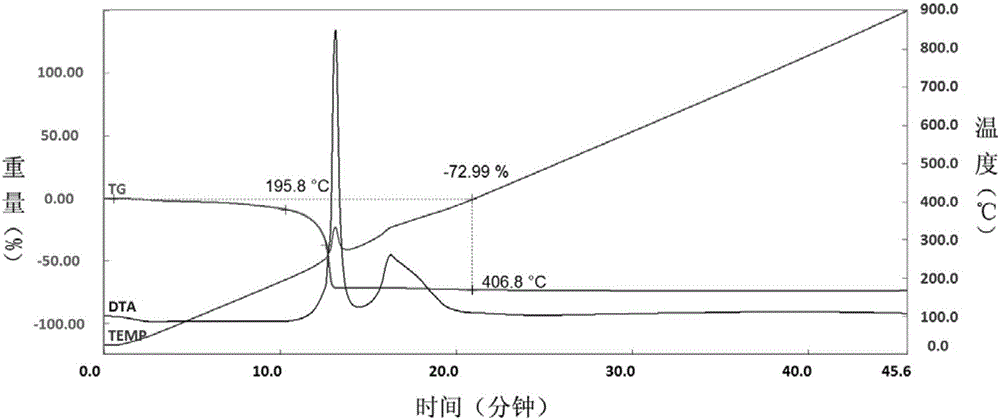
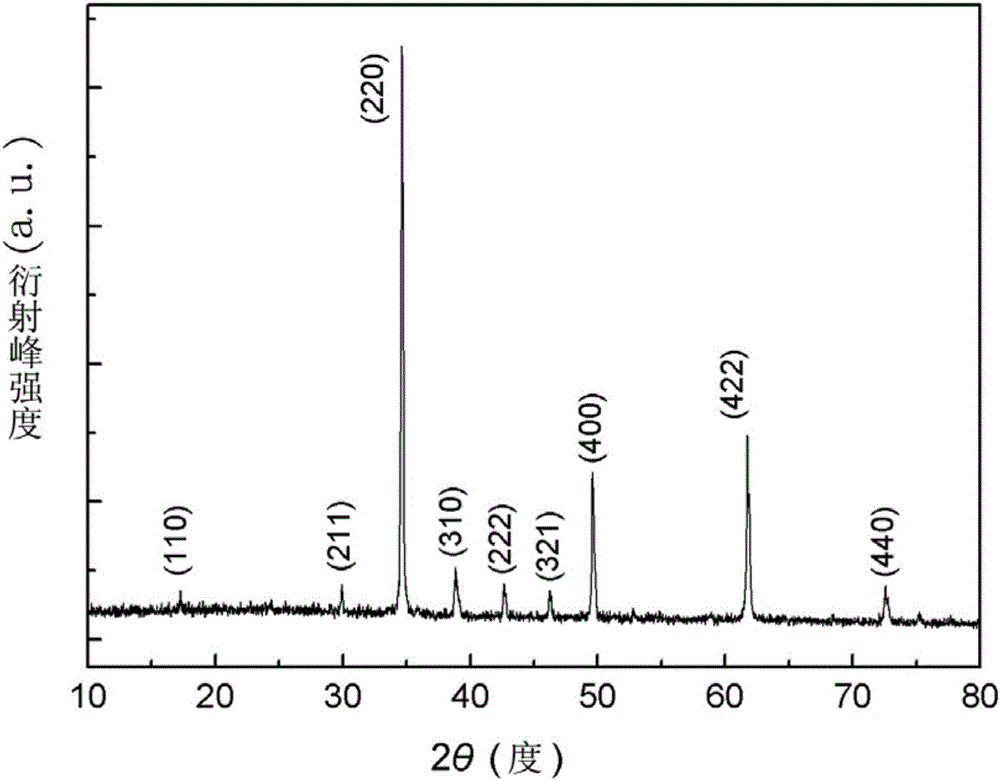
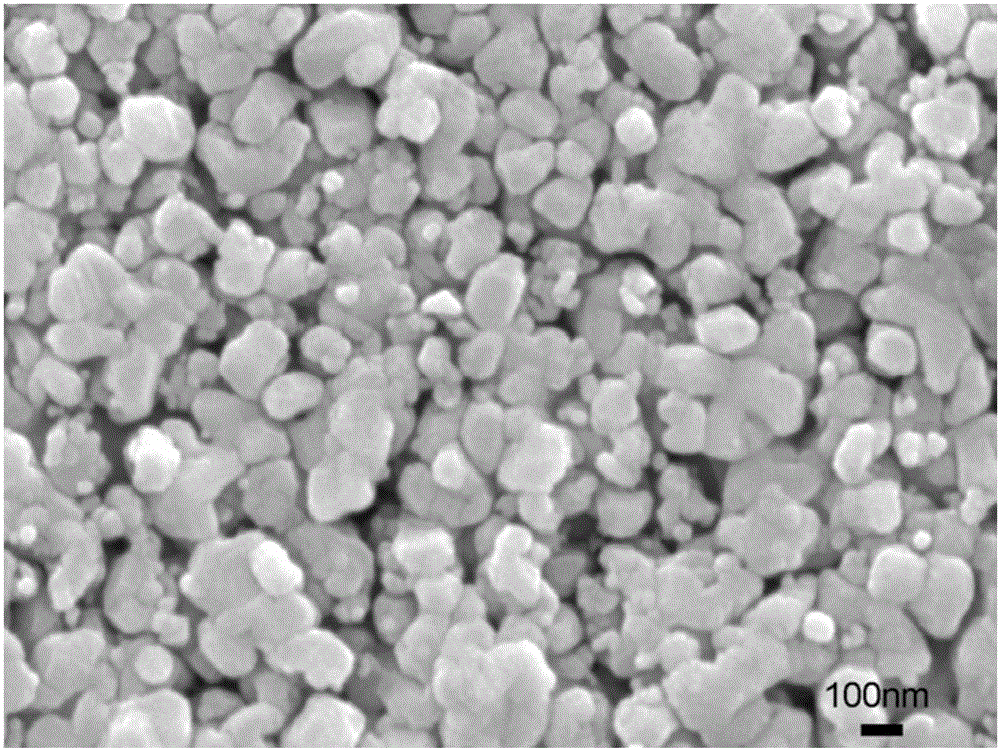
[0036] (4) Fully grind the light blue needle-like crystals, then raise the temperature to 450° C. at a r...
Embodiment 2
[0045] The preparation method of the present embodiment CCTO crystal powder is based on calcium acetylacetonate Ca (acac) 2 , Copper acetylacetonate Cu(acac) 2 and titanyl acetylacetonate TiO(acac) 2 As a raw material, using absolute ethanol as a solvent and anhydrous citric acid as a stabilizer, the CCTO crystal powder is obtained by low-temperature calcination, which specifically includes the following steps:
[0046] (1) Weigh 0.01mol of Ca(acac) 2 , 0.033mol of Cu(acac) 2 and 0.04mol of TiO(acac) 2 , add 20ml of absolute ethanol, and stir vigorously with a magnetic stirrer to obtain a mixed solution;
[0047] (2) Add anhydrous citric acid to the mixed solution to adjust the pH value to 1.8 to obtain a light blue solution, and continue to stir for 0.5 h;
[0048] (3) drying the solution in step (2) at 85° C. for 15 hours to obtain light blue needle-like crystals;
[0049] (4) Fully grind the light blue needle-like crystals, then raise the temperature to 500° C. at a r...
Embodiment 3
[0052] The preparation method of the present embodiment CCTO crystal powder is based on calcium acetylacetonate Ca (acac) 2 , Copper acetylacetonate Cu(acac) 2 and titanyl acetylacetonate TiO(acac) 2 As a raw material, using absolute ethanol as a solvent and anhydrous citric acid as a stabilizer, the CCTO crystal powder is obtained by low-temperature calcination, which specifically includes the following steps:
[0053] (1) Weigh 0.01mol of Ca(acac) 2 , 0.035mol of Cu(acac) 2 and 0.04mol of TiO(acac) 2 , add 20ml of absolute ethanol, and stir vigorously with a magnetic stirrer to obtain a mixed solution;
[0054] (2) Add anhydrous citric acid to the mixed solution to adjust the pH value to 1.2 to obtain a light blue solution, and continue to stir for 1 hour;
[0055] (3) drying the solution in step (2) at 90° C. for 10 h to obtain light blue needle-like crystals;
[0056] (4) Fully grind the light blue needle-like crystals, then raise the temperature to 600° C. at a rate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com