Method for preparing moxifloxacin side chain through biological method
A technology of compound and transaminase, which is applied in the production of bulk chemicals, organic chemistry, fermentation, etc., can solve the problems of long nonane steps, high environmental pressure, and unknown enzyme sources, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Screening of amino protecting groups
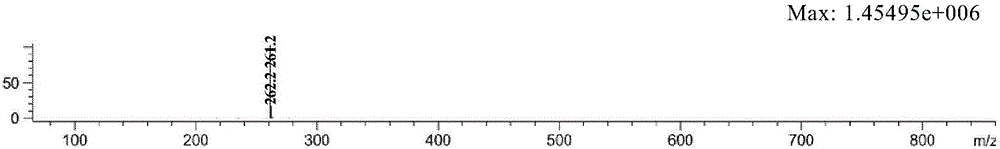
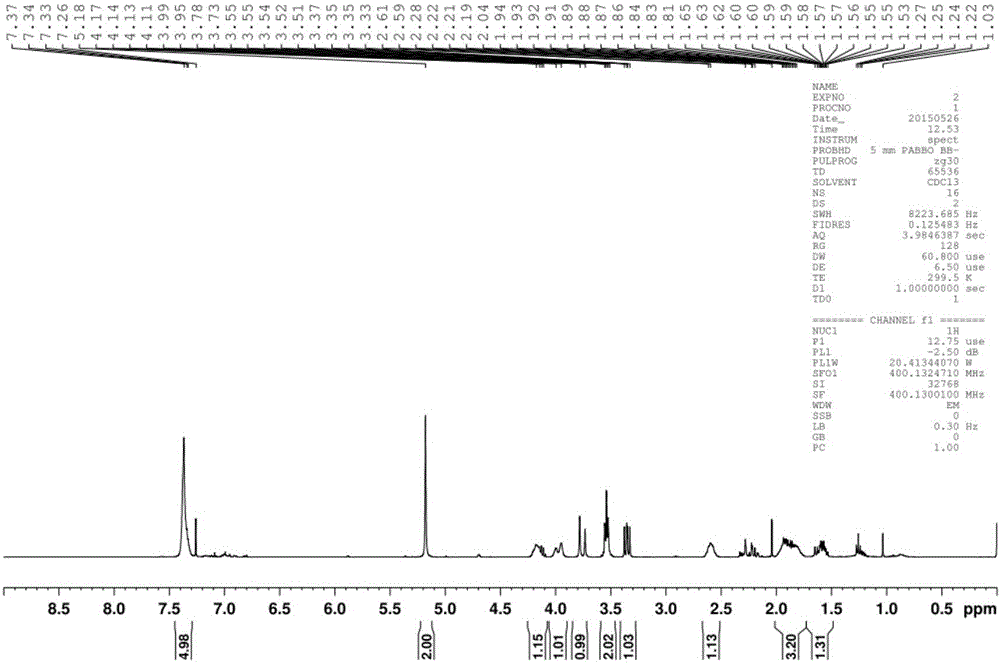
[0065] The substrates were 10 mg of 4-(3-chloropropyl)-3-pyrrolidone (4a, 4b, 4c) protected by benzyl group, ethyl formate group and Cbz group, dissolved in 0.2 mL DMSO, and added to 0.8 mL In triethanolamine buffer solution (pH8.0, 0.1M), add transaminase (SEQ ID NO: 18) 10mg, pyridoxal phosphate (PLP) 1mg, isopropylamine 0.01mL, react at 30 degrees for 24 hours, and detect the conversion rate> 99%, adding 1mL ethyl acetate to extract 5 times, combining the organic phases, drying and concentrating to obtain products 2a, 2b, and 2c were 3.44mg, 7.44mg, and 8.37mg respectively, and the molar yields were 40%, 88%, and 95% respectively. %.
[0066]
Embodiment 2
[0067] Embodiment 2: Investigation on the stability of benzyl-protected substrates
[0068] The stability of the benzyl-protected substrate was investigated due to possible problems with the stability of the substrate. Substrate benzyl-protected 4-(3-chloropropyl)-3-pyrrolidone (4a) 10mg, dissolved in 0.2mL DMSO, respectively added 0.8mL triethanolamine buffer solution (pH 8.0, 0.1M), phosphate buffer solution (pH 8.0, 0.1M) and deionized water, add 0.01mL of isopropylamine, shake at 30 degrees for 24 hours, take a sample to detect the change of the peak area of the substrate in HPLC, as shown in the following table:
[0069]
[0070] It shows that the substrate is unstable under the enzyme reaction conditions.
Embodiment 3
[0071] Embodiment 3: reaction temperature selection
[0072] Dissolve 10 mg of substrate 4c in 0.2 mL DMSO, add 0.8 mL of triethanolamine buffer solution (pH 8.0, 0.1 M), add 1 mg of transaminase (SEQ ID NO: 18), 0.1 mg of PLP, 0.01 mL of isopropylamine, different Reaction under the temperature for 24 hours, detection conversion rate, the results are shown in the table below:
[0073]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com