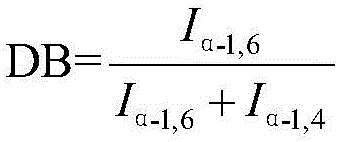Preparation method of icodextrin for starch-based peritoneal dialysis solution
A peritoneal dialysate and icodextrin technology, applied in the field of starch dextrin preparation, can solve the problems of large measurement method error, α-1,6 glycosidic bond ratio control, no specific method for icodextrin preparation, etc. , to achieve the effect of narrow distribution range and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
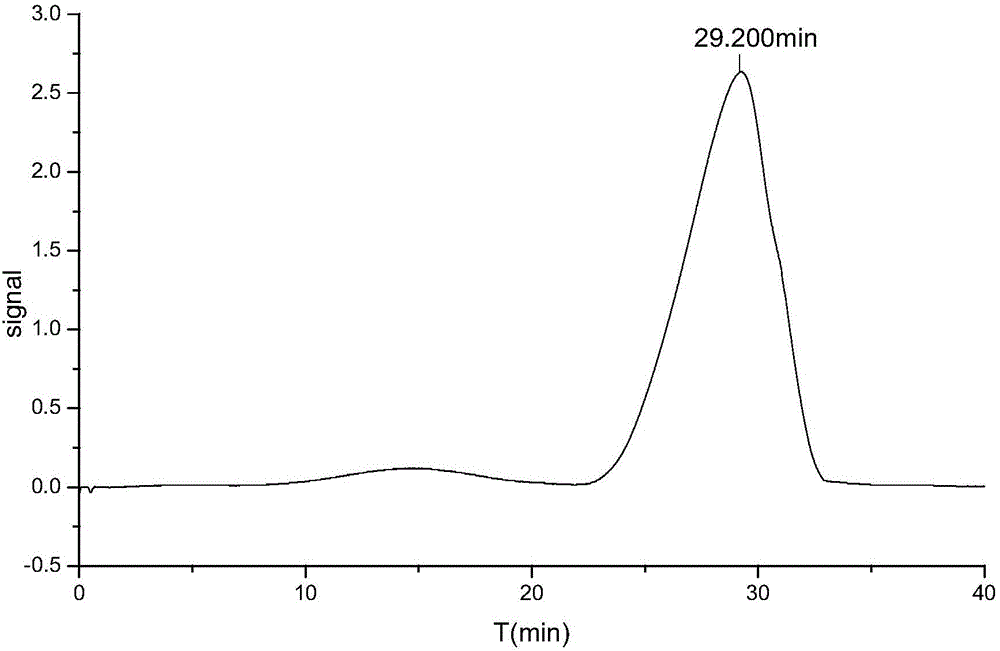
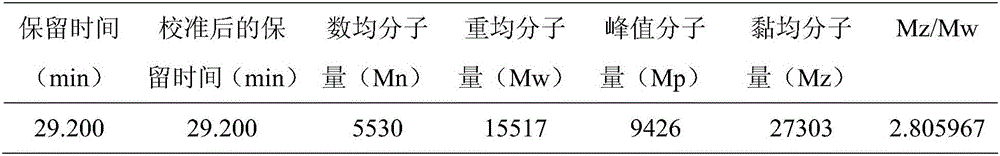
Image
Examples
Embodiment 1
[0035](1) Prepare cornstarch with phosphate buffer solution to form a starch solution with a mass percent concentration of 15%, and adjust the pH to 7.8, place it in an airtight container, and stir and gelatinize at 99°C for 60 minutes; after gelatinization is completed, cool To 95°C, add high temperature resistant α-amylase (Liquozyme Supra, Novozymes company), the dosage is 8 U of high temperature resistant α-amylase per gram of dry starch, enzymatic hydrolysis for 1 min, adjust the pH to 2.5, and keep for 10 min to inactivate the enzyme activity, obtain the starch solution after enzymolysis;
[0036] (2) Add pullulanase (OPTIMAX L-1000, Genencor Bioengineering Co., Ltd.) to the starch solution after enzymolysis in step (1) at a pH of 5.5 and 60°C, and the dosage is 1 gram of dry starch Add 10 U of pullulanase, stir and react for 30 minutes; heat to 100°C for 10 minutes to inactivate the enzyme activity, and obtain the crude enzymatic hydrolyzate of peritoneal dialysate;
...
Embodiment 2
[0045] (1) Prepare wheat starch with a phosphate buffer solution to form a starch solution with a mass percent concentration of 5%, and adjust the pH to 5.8, place it in an airtight container, and stir and gelatinize at 95°C for 100 minutes; after the gelatinization is completed, cool To 70°C, add high temperature resistant α-amylase (Liquozyme Supra, Novozymes company), the dosage is 1 U of high temperature resistant α-amylase per gram of dry starch, enzymatic hydrolysis for 8 min, adjust the pH to 3.0, and keep for 15 min to inactivate the enzyme activity, obtain the starch solution after enzymolysis;
[0046] (2) Add isoamylase (Pseudomonas sp, Sigma-Aldrich company) to the starch solution after enzymolysis in step (1) at a pH of 6.5 and 50° C., in an amount of 2 U of isoamylase per gram of dry starch, Stir the reaction for 30 minutes; heat to 90°C for 15 minutes to inactivate the enzyme, and obtain the crude enzymatic hydrolyzate of the peritoneal dialysate;
[0047] (3) ...
Embodiment 3
[0052] (1) Prepare the waxy cornstarch with a phosphate buffer solution to form a starch solution with a mass percentage concentration of 5%, and adjust the pH to 7.0, place it in an airtight container, and stir and gelatinize at 95°C for 100 minutes; after the gelatinization is completed , cooled to 95°C, added high temperature resistant α-amylase (Liquozyme Supra, Novozymes company), the dosage was 8 U of high temperature resistant α-amylase per gram of dry starch, enzymatic hydrolysis for 3 minutes, adjusted pH to 2.7, and kept for 12 minutes to kill the enzyme live to obtain the starch solution after enzymolysis;
[0053] (2) Add pullulanase (OPTIMAX L-1000, Genencor Bioengineering Co., Ltd.) to the starch solution after enzymolysis in step (1) at a pH of 5.8 and 40°C, and the dosage is 1 gram of dry starch Add 8 U of pullulanase, stir for 40 minutes to react; heat to 95°C for 12 minutes to inactivate the enzyme activity, and obtain crude enzymatic hydrolyzate of peritonea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com