Preparation method of manganese-doped fluoride luminescent material controllable in morphology and particle size
A technology of luminescent materials and manganese doping, which is applied in the direction of luminescent materials, chemical instruments and methods, etc. It can solve problems such as difficult to achieve continuous production in large quantities, volatilization of HF, and difficulty in controlling the shape of product particles.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
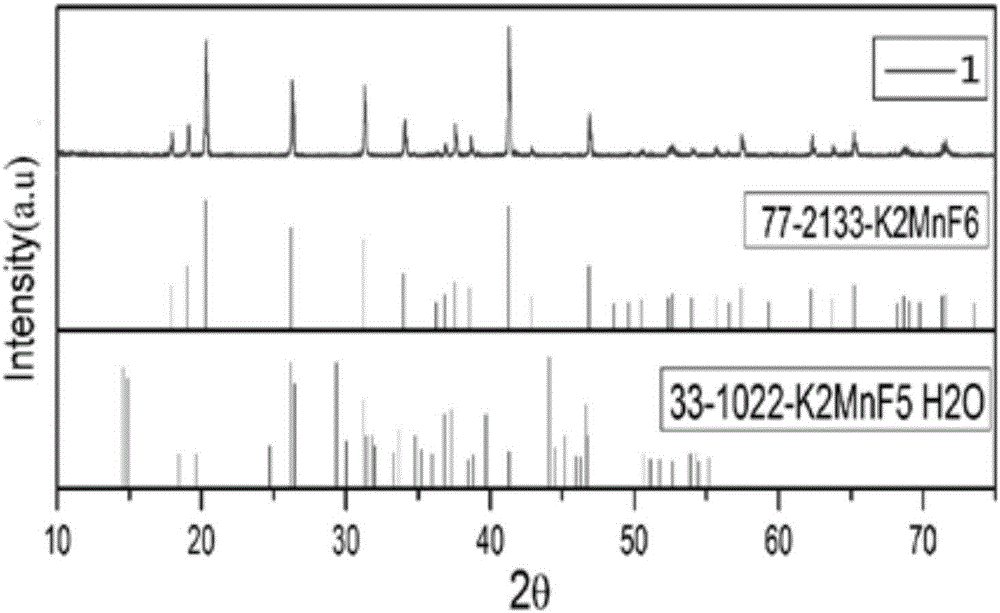
[0046] Preparation of tetravalent manganese ions: Weigh the required hydrofluoric acid solution with a graduated cylinder, and weigh the calculated amount of fluoride containing A and KMnO 4 Make it slowly dissolve in the hydrofluoric acid solution, control the reaction temperature to be less than 20°C, slowly add 30wt% H 2 o 2 Solution, when the color of the bottom solution starts to change from purple to golden yellow, stop adding H 2 o 2 , adopt the solid-liquid separation method to extract the required precipitate, and obtain the yellow powder K containing tetravalent manganese ions 2 MnF 6 ;
[0047] The first step is to prepare the original crystal of manganese-doped fluoride luminescent material with a particle size of 2 MnF 6 Dissolved in hydrofluoric acid as the bottom liquid, control the synthesis temperature. The compound containing M and or N is weighed and dissolved in an appropriate amount of hydrofluoric acid as a drop solution. Stir the bottom liquid and...
Embodiment 1
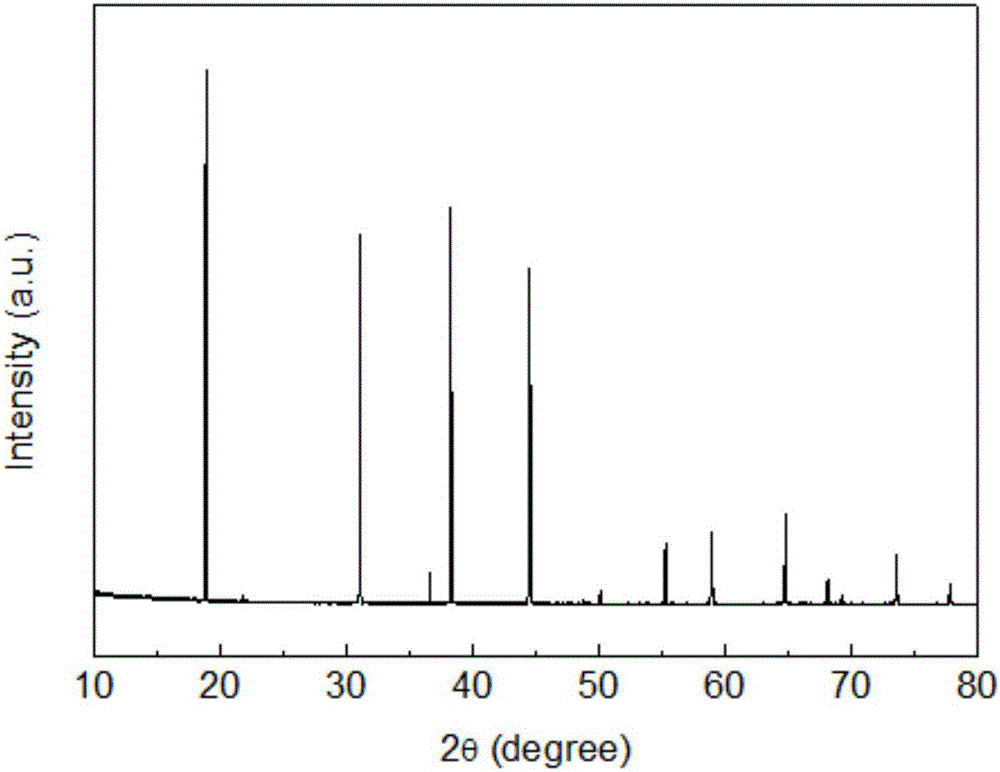
[0057] Two-step preparation of spherical K with controllable morphology and particle size 2 Ge 0.95 f 6 : 0.05 mn 4+ Fluoride red luminescent material
[0058] In a polytetrafluoro beaker, weigh 100ml of 40wt% hydrofluoric acid solution in a polytetrafluoro beaker with a measuring cylinder, and weigh 30.00gKHF 2 and 1.50 g KMnO 4 It is slowly dissolved in hydrofluoric acid solution, and the temperature is controlled within the range of 25-30°C. Place the polytetrafluoro beaker in the water bath of the cooler and start to cool down to below 20°C, slowly add 30wt% H 2 o 2 Solution, the color of the bottom solution starts to change from purple to golden yellow, stop adding H 2 o 2 , after a stable reaction for 15 minutes, filter with suction, rinse with a small volume of acetone three times, put the filter cake in an oven and dry at 100°C for 2 hours, break it through a 100-mesh sieve at room temperature, and obtain K 2 MnF 6 Yellow powder ready for use;
[0059] Accu...
Embodiment 2
[0065] Two-step preparation of spherical K with controllable morphology and particle size 2 Ti 0.95 f 6 : 0.05 mn 4+ Fluoride red luminescent material
[0066] Accurately weigh 10ml of 49wt% HF solution and place it in a Teflon beaker, weigh 5.00g of KHF 2 and 0.16g K 2 MnF 6 Dissolve in hydrofluoric acid and control the temperature to 25°C as the reaction bottom solution. Weigh 1.00g TiO2 2 Dissolve in 5ml of 49wt% HF as a drop solution, control the temperature of the drop solution to 25°C, stir the bottom solution, add the drop solution dropwise to the bottom solution, and stir for 30 minutes after the dropwise addition. The color of the reaction bottom solution gradually changed from initial golden yellow to light, and precipitates were precipitated during the process. Suction filter the bottom liquid of the reaction, rinse it with a small volume of acetone three times, then place it in an oven at 100°C and dry it for 2 hours, take out the dry cake and crush it thr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com