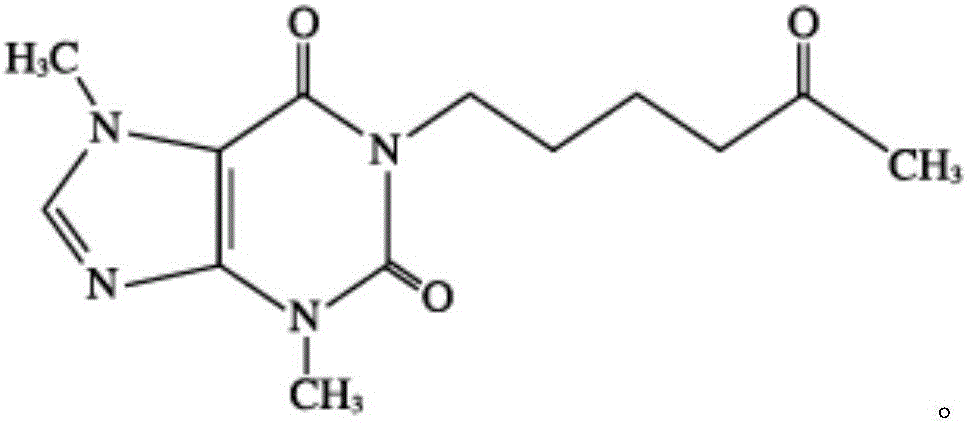
Pentoxifylline injection composition and preparation method thereof
A technology of theobromine injections and compositions, which is applied in the direction of drug combinations, active ingredients of heterocyclic compounds, anti-inflammatory agents, etc., can solve problems such as poor stability, adverse reactions, and increased content of related substances, and achieve good quality stability and process Simple and easy to implement, enhanced stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 Pentoxifylline injection composition of the present invention
[0030] prescription:
[0031]
[0032] Preparation Process:
[0033] Take 70% of the total amount of water for injection at 75°C, add sodium dihydrogen phosphate, taurine, and vitamin B6 to dissolve, then add disodium edetate, add pentoxifylline, stir to dissolve, and then add 2% phosphoric acid Adjust the pH value of the disodium hydrogen solution to 5.0, add activated carbon for needles with a total volume of 0.1% (g / mL), stir and adsorb at 50-60°C for 20 minutes, filter and decarburize with a titanium filter stick, add water for injection to 1L, and stir Evenly, after coarse filtration and fine filtration with a 0.22um filter, filled with nitrogen, potted and sealed, sterilized with damp heat at 100°C for 30 minutes to obtain the product.
Embodiment 2
[0034] Embodiment 2 Pentoxifylline injection composition of the present invention
[0035] prescription:
[0036]
[0037] Preparation Process:
[0038] Take 80% of the total amount of water for injection at 80°C, add sodium dihydrogen phosphate, taurine, and vitamin B6 to dissolve, then add disodium edetate, add pentoxifylline, stir to dissolve, and then add 2% phosphoric acid Adjust the pH value of the disodium hydrogen solution to 5.5, add activated carbon for needles with 0.1% (g / mL) of the total volume of the solution, stir and absorb at 50-60°C for 20 minutes, filter and decarburize with a titanium filter stick, add water for injection to 1L, and stir Evenly, after coarse filtration and fine filtration with a 0.22um filter, filled with nitrogen, potted and sealed, sterilized with damp heat at 100°C for 30 minutes to obtain the product.
Embodiment 3
[0039] Embodiment 3 Pentoxifylline injection composition of the present invention
[0040] prescription:
[0041]
[0042]
[0043] Preparation Process:
[0044] Take 75% of the total amount of water for injection at 80°C, add sodium dihydrogen phosphate, taurine, and vitamin B6 to dissolve, then add disodium edetate, add pentoxifylline, stir to dissolve, and then add 2% phosphoric acid Adjust the pH value of the disodium hydrogen solution to 5.6, add activated carbon for needles with a total volume of 0.1% (g / mL), stir and adsorb at 50-60°C for 20 minutes, filter and decarburize with a titanium filter stick, add water for injection to 1L, and stir Evenly, after coarse filtration and fine filtration with a 0.22um filter, filled with nitrogen, potted and sealed, sterilized with damp heat at 100°C for 30 minutes to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com