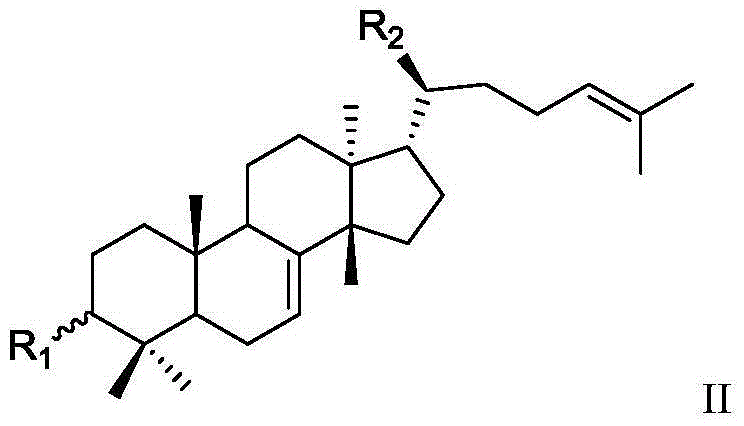
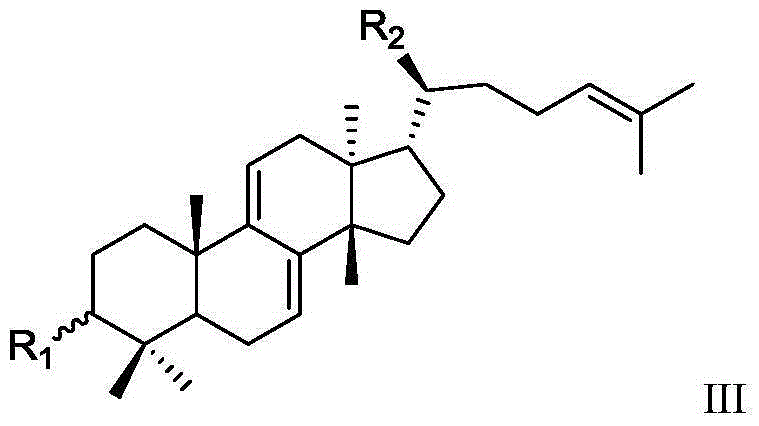
Mastic tetracyclic triterpenoid components with 11 beta-hydroxysteroid dehydrogenase 1 inhibitory activity, composition of mastic tetracyclic triterpenoid components and application
A technology of hydroxysteroids and dehydrogenase inhibitors, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 11β-HSD inhibitory activity test
[0044] 1. Experimental materials
[0045] Reagents: Boswellia tetracyclic triterpenes, all self-made by our research group, with a purity greater than 95%; cortisone, hydrocortisone, prednisone acetate and nicotinamide adenine dinucleotide phosphate (NADPH) were purchased from Bailingwei Reagent Company ; Methanol is chromatographically pure for HPLC, and the rest of the reagents are analytical grade domestic reagents.
[0046] Animals: Wistar rats, weighing 240-300 g, were bred and raised by the animal room of Tianjin Institute of Pharmaceutical Research.
[0047] Instruments: 515 type high performance liquid chromatography pump (Waters Company), 717plus autosampler (Waters Company), 2487 type ultraviolet detector (Waters Company).
[0048] 2. Experimental method:
[0049] 2.1 Enzyme activity inhibition experiment
[0050] Preparation of liver microsomes and analysis of 11β-HSD1 activity: several rats were taken, anesthetized with...
Embodiment 2
[0062] Take 300g of frankincense medicinal material, put it in a 2L round bottom flask, add 1.5L of 95% ethanol, heat and reflux for extraction for 2hr, 1.5hr, 1.5hr. The extracts were filtered and combined. Concentrate the extract under reduced pressure until there is no alcohol smell to obtain liquid extract. Disperse it in 400mL water, adjust the pH value to 9-10 with 2% NaOH solution, extract with ethyl acetate, and separate the organic layer; continue to adjust the pH value of the aqueous layer to 3-4 with dilute hydrochloric acid, extract three times with ethyl acetate, and combine The organic layer was washed with water until neutral, dehydrated with anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 56.8 g of liquid extract.
[0063] Dissolve the above liquid extract with a small amount of methanol, mix the sample with coarse silica gel, and load the sample on a 500g column chromatography silica gel column. Gradient elution with petroleum eth...
Embodiment 3
[0068] Take 300g of frankincense medicinal material, put it in a 2L round bottom flask, add 1.5L of 95% ethanol, heat and reflux for extraction for 2hr, 1.5hr, 1.5hr. The extracts were filtered and combined. Concentrate the extract under reduced pressure until there is no alcohol smell to obtain liquid extract. Disperse it in 400mL water, adjust the pH value to 9-10 with 2% NaOH solution, extract with ethyl acetate, and separate the organic layer; continue to adjust the pH value of the aqueous layer to 3-4 with dilute hydrochloric acid, extract three times with ethyl acetate, and combine The organic layer was washed with water until neutral, dehydrated with anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 56.8 g of liquid extract.
[0069] Dissolve the above liquid extract with a small amount of methanol, mix the sample with coarse silica gel, and load the sample on a 500g column chromatography silica gel column. Gradient elution with petroleum eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com