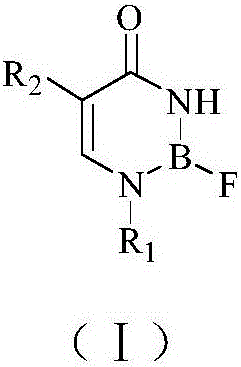
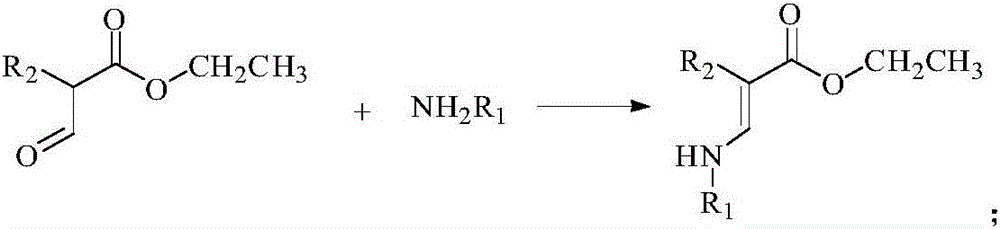Boron-containing compound for BNCT and preparation method and application thereof
A boron compound and the technology of the compound are applied in the fields of medicine and tumor-related drugs, which can solve the problems of false positive brain tumor imaging effect and the like, and achieve the effects of good application prospect, low cost and easy preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 compound Ia
[0052] reaction route
[0053]
[0054] 1.1 Preparation of Intermediate 3a
[0055] Add 1 (13.00 mg, 0.1 mmol) and 2a (27.53 mg, 0.1 mmol) into a 50 mL reaction flask, add 20 mL of methanol solution and stir at room temperature for 24 hours, stop the reaction, and obtain 3a (34.87 mg, 90%) by suction filtration .
[0056] 1 H NMR (500MHz, CDCl 3 ): δppm 5.49 (1H, CH), 5.13 (1H, CH), 4.94 (1H, CH), 4.89 (1H, CH), 4.23 (2H, CH 2 ), 4.16 (2H, CH 2 ), 1.99 (9H, CH 3 ), 2.01 (1H, NH), 1.91 (3H, CH 3 ), 1.27 (3H, CH 3 ).
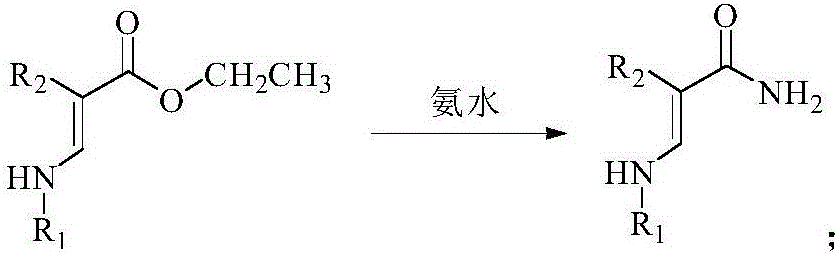
[0057] 1.2 Preparation of Intermediate 4a
[0058] Add 3a (38.74mg, 0.1mmol) and concentrated ammonia water (30.36mg, 0.5mmol) into a 50mL reaction flask, add 20mL of methanol solution, stir at room temperature for 1h, then raise the temperature to reflux for 6h, cool down and suction filter to obtain 4a (28.31 mg, 79%).
[0059] 1 H NMR (500MHz, CDCl 3 ): δppm 6.67 (1H, CH), 5.96 (2H, NH 2 ), ...
Embodiment 2
[0063] The preparation of embodiment 2 compound Ib
[0064] reaction route
[0065]
[0066] 2.1 Preparation of compound 3b
[0067] Add 1 (13.00mg, 0.1mmol) into a 50mL reaction flask, add 20mL of methanol solution and stir at room temperature, pass through ammonia gas, stop the reaction when the reaction is no longer monitored by TLC, and filter to obtain 3b (11.11mg, 86%).
[0068] 1 H NMR (500MHz, CDCl 3 ): δppm 6.97 (1H, CH), 4.18 (2H, CH 2 ), 2.01 (2H, NH 2 ), 1.91 (3H, CH 3 ), 1.27 (3H, CH 3 ).
[0069] 2.2 Preparation of compound 4b
[0070] Add 3b (12.92mg, 0.1mmol) and concentrated ammonia water (30.36mg, 0.5mmol) into a 50mL reaction flask, add 20mL of methanol solution, stir at room temperature for 1h, then raise the temperature to reflux for 6h, cool down and suction filter to obtain 4b (8.11 mg, 81%).
[0071] 1 H NMR (500MHz, CDCl 3 ): δppm 6.68 (1H, CH), 6.01 (2H, NH 2 ), 2.01 (2H, NH 2 ), 1.91 (3H, CH 3 ).
[0072] 2.3 Preparation of Compou...
Embodiment 3
[0075] The preparation of embodiment 3 compound IIa
[0076] reaction route
[0077]
[0078] 3.1 Preparation of intermediate 5a
[0079] Add Ia (38.61mg, 0.1mmol) and 10% NaOH solution (160mg, 0.4mmol) to a 50mL reaction flask, raise the temperature to 50°C and react for 6h, cool down to room temperature after the reaction, extract twice with ethyl acetate, concentrate , and recrystallized from methanol to give 5a (17.54mg, 68%).
[0080] 1H NMR (500MHz, CDCl 3 ): δppm 8.02 (1H, NH), 6.27 (1H, CH), 4.98 (1H, CH), 3.92 (1H, CH), 3.89 (1H, CH), 3.67 (2H, CH 2 ), 3.65 (1H, CH) 2.02 (4H, OH), 1.92 (3H, CH 3 ).
[0081] 3.2 Preparation of compound Ⅱa
[0082] with 15mg K 222 and 3 mg K 2 CO 3 Acetonitrile water rinse 18 f - For the enriched QMA column, after acetotropic water removal, use K 2 CO 3 , K 222 , [ 18 F]-F -The mixture of and the labeled precursor compound 5a are reacted in an acetonitrile solution under heating conditions, the reaction temperature is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com