Method for removing bacteria from blood using high flow rate
A technology of bacteria and bacterial infection, which can be applied in blood circulation treatment, medical equipment, and disease resistance to vector-borne diseases, and can solve problems such as the increase in the incidence of sexual CRE
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1. Removal of bacteria with low or undetectable affinity for heparan sulfate
[0109] This example illustrates the use of heparin-coated beads to remove bacterial pathogens with low or undetectable affinity for heparan sulfate from whole blood.
[0110] It has been reported in the literature that more than 50 different pathogens target heparan sulfate proteoglycan on syndecan as the initial binding site during their pathology. Surprisingly, surface-bound heparin can function as a substitute for organism-bound heparan sulfate.
[0111] Our studies have shown that heparinized adsorption media can remove high concentrations of S. aureus and MRSA from whole blood. In addition, studies have shown that bacteria bound to the heparinized surface are not killed and thus do not release potential inflammatory toxins and their by-products into the blood. Thus, heparin-conjugated mediators can be used in extracorporeal devices to efficiently and safely remove circulating b...
Embodiment 2
[0141] Example 2. Adsorption media with a hydrophilic surface
[0142] This example shows that adsorption media comprising a hydrophilic surface can be used to remove bacteria from whole blood or serum.
[0143] The adsorption media described herein contain surface topography that enables them to bind pathogens such as those that have no or low affinity for heparin ( Figure 1A ). Without being bound by any particular theory, it is believed that rough, uneven or wavy surfaces may facilitate the affinity of bacteria to the adsorption medium.
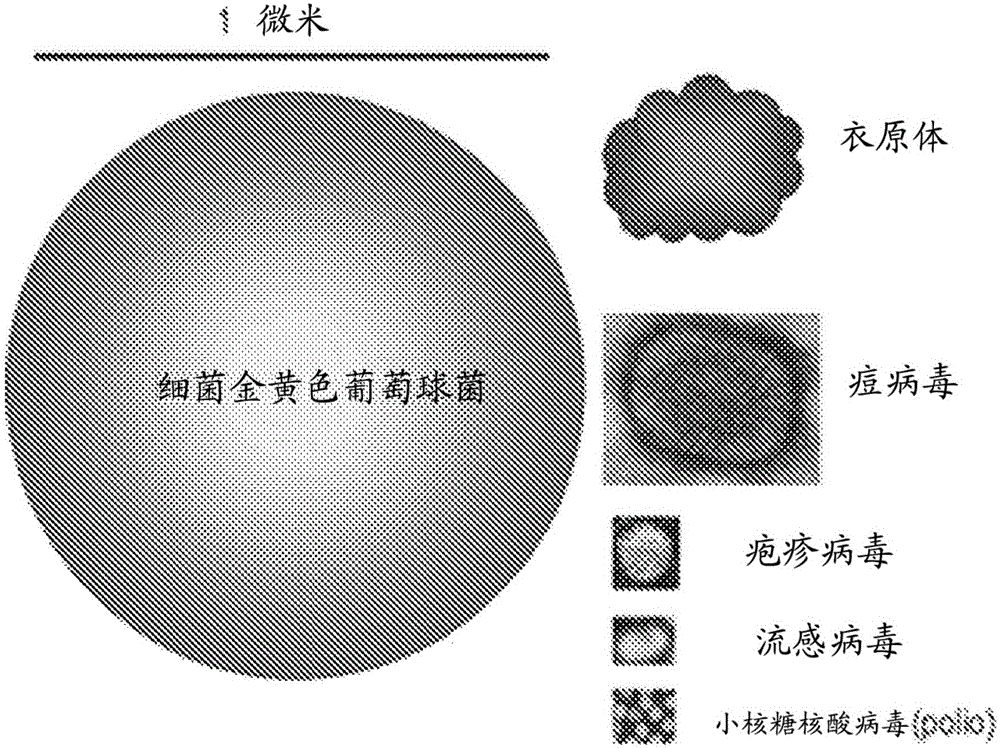
[0144] Figure 1B Human blood smear images are shown for comparison. figure 2 A size comparison is shown for bacteria (eg, Staphylococcus aureus and Chlamydia) and viruses (eg, poxviruses, herpesviruses, influenza viruses, and picornaviruses (polioviruses)).
Embodiment 3
[0145] Example 3: Blood filter for high linear flow rate extracorporeal therapy
[0146] This example provides an exemplary design for an extracorporeal filter cartridge to accommodate high linear flow rates.
[0147] Extracorporeal blood filters can be designed to operate safely at the specific flow rates used by common pump systems. If the pressure drop across the blood filter is too great, hemolysis may occur. Typically, dialysis systems are operated using pressures below 34 kPa to avoid the risk of hemolysis.
[0148] For a cartridge filled with packed sorbent media, the pressure drop across the cartridge depends on the flow rate, particle size, particle module, height of the packing medium, and viscosity of the blood. If the filter media is not rigid enough, then with increased blood flow, compaction of the media may occur, resulting in reduced porosity, which may create unsafe pressures.
[0149] The first variable to be determined is the smallest particle size that c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com