Method for preparing 1-amino-anthraquinone from 1-nitro-anthraquinone by catalytic hydrogenation
A technology of nitroanthraquinone catalysis and aminoanthraquinone, applied in the direction of chemical instruments and methods, preparation of organic compounds, physical/chemical process catalysts, etc., can solve problems such as retention, high production cost, and no industrial value, and achieve Less investment in equipment, improved catalytic performance, and environmentally friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Catalyst preparation:
[0028] In the presence of organic modifier polyethylene glycol 600, copper nitrate trihydrate, nickel acetate tetrahydrate and hydrazine hydrate were used as raw materials to prepare nano-copper / nickel binary alloy catalysts by wet chemical reduction method.
[0029] First take by weighing 3.2g of copper nitrate trihydrate and 1.3g of nickel acetate tetrahydrate and dissolve them in 50ml of absolute ethanol respectively, and mix 20ml of aqueous solution with 0.32g of polyethylene glycol 600 and the ethanol solution of copper and nickel; Under stirring, at a constant temperature of 60° C., 1.5 mol / L NaOH ethanol solution was added dropwise to adjust the pH value of the solution to 13. Then measure 20ml of hydrazine hydrate with a mass fraction of 85% and dilute it to 100ml with absolute ethanol, add dropwise to the above solution, and prepare nano-copper-nickel binary alloy Cu / Ni after reacting at 80°C for 4h, and cool to room temperature and sto...
Embodiment 2
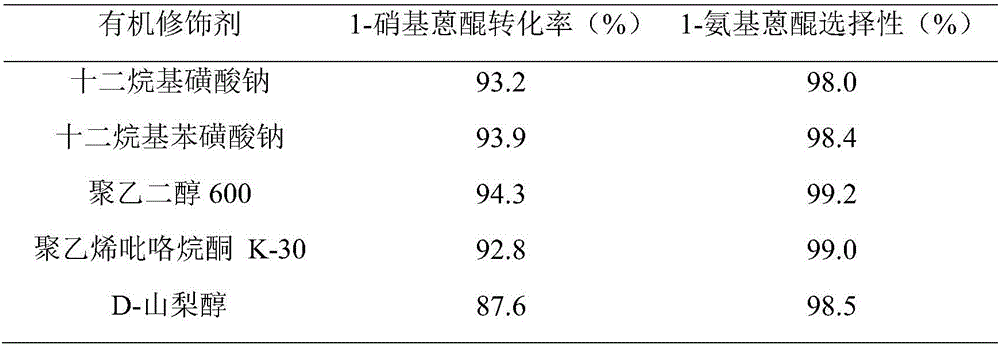
[0033] Adopt the same catalyst preparation method of Example 1, only change the organic modifier to be polyvinylpyrrolidone K-30 or sodium dodecylsulfonate or D-sorbitol or sodium dodecylbenzenesulfonate to prepare nano-copper-nickel di Elemental alloy Cu / Ni. The catalytic hydrogenation reaction of 1-nitroanthraquinone adopts the same method as in Example 1 to obtain the influence of different organic modifiers on the catalytic hydrogenation reaction of 1-nitroanthraquinone prepared by nano-copper-nickel catalysts. The results are shown in Table 1.
[0034] Table 1 Effect of Cu / Ni nanocatalysts prepared by different organic modifiers on the catalytic hydrogenation of 1-nitroanthraquinone
[0035]
Embodiment 3
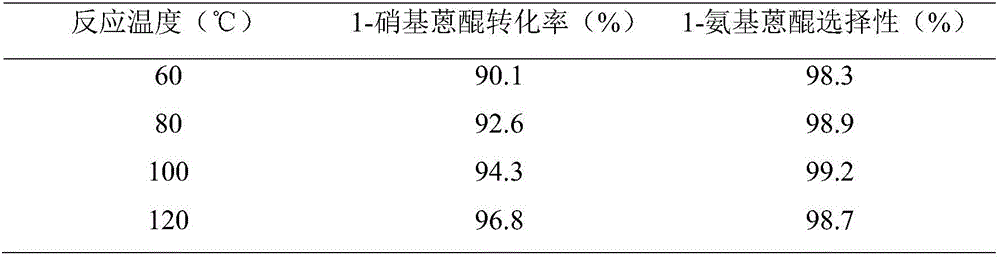
[0037] Using the same method as in Example 1 to prepare nano-Cu / Ni catalysts, catalyze the hydrogenation of 1-nitroanthraquinone, and only change the reaction temperature in the kettle to 60 ° C, 80 ° C and 120 ° C respectively, the reaction temperature can be obtained. The influence of nitroanthraquinone catalytic hydrogenation reaction, the results are shown in Table 2.
[0038] The influence of table 2 reaction temperature on 1-nitroanthraquinone catalytic hydrogenation reaction
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com