Network polymer material applied to super capacitor electrode and provided with nitride and oxygen atoms and preparation method thereof
A technology of polymer materials and supercapacitors, applied in the field of material science, can solve the problems of few effective active sites and uneven distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] In this example, MHCF@600 was prepared through coupling reaction and polymerization reaction.
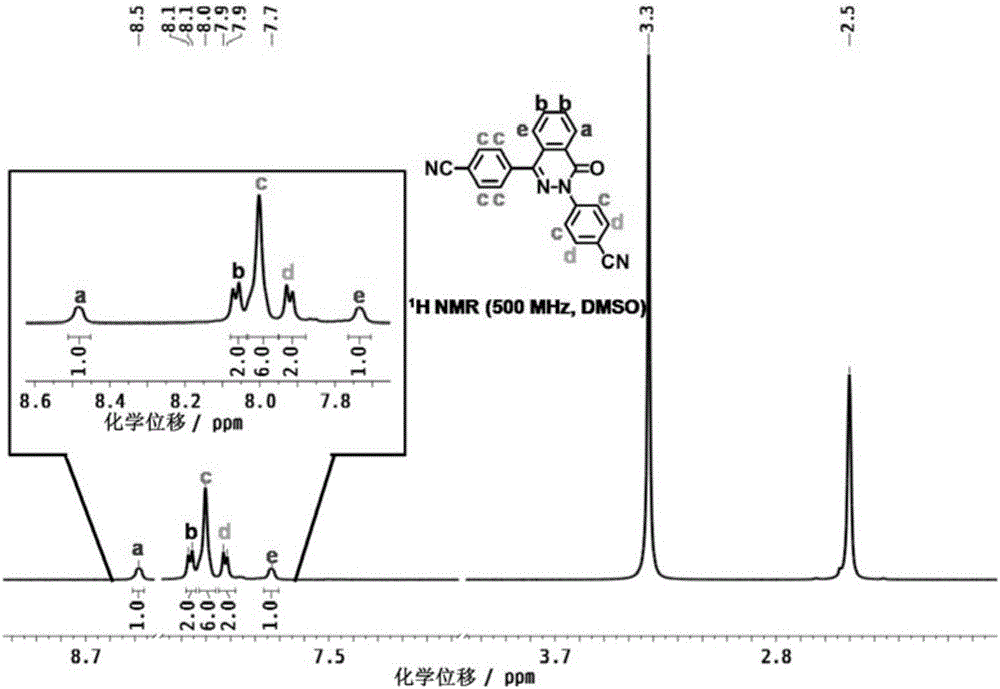
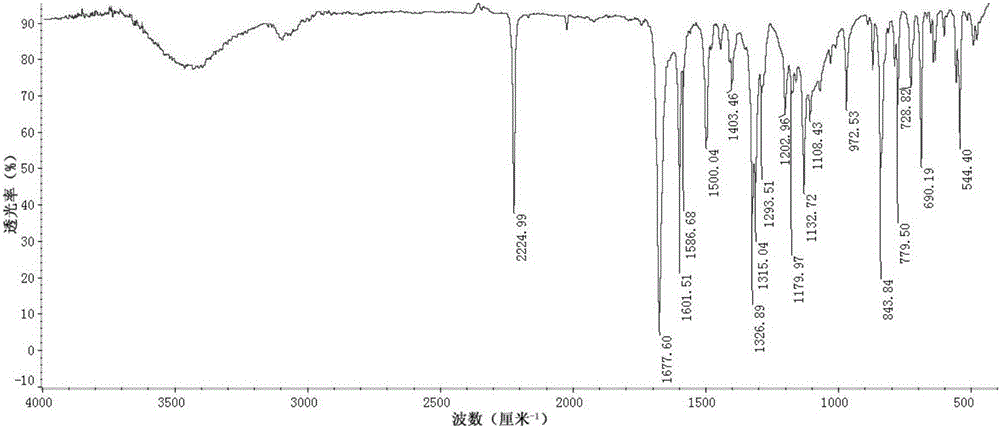
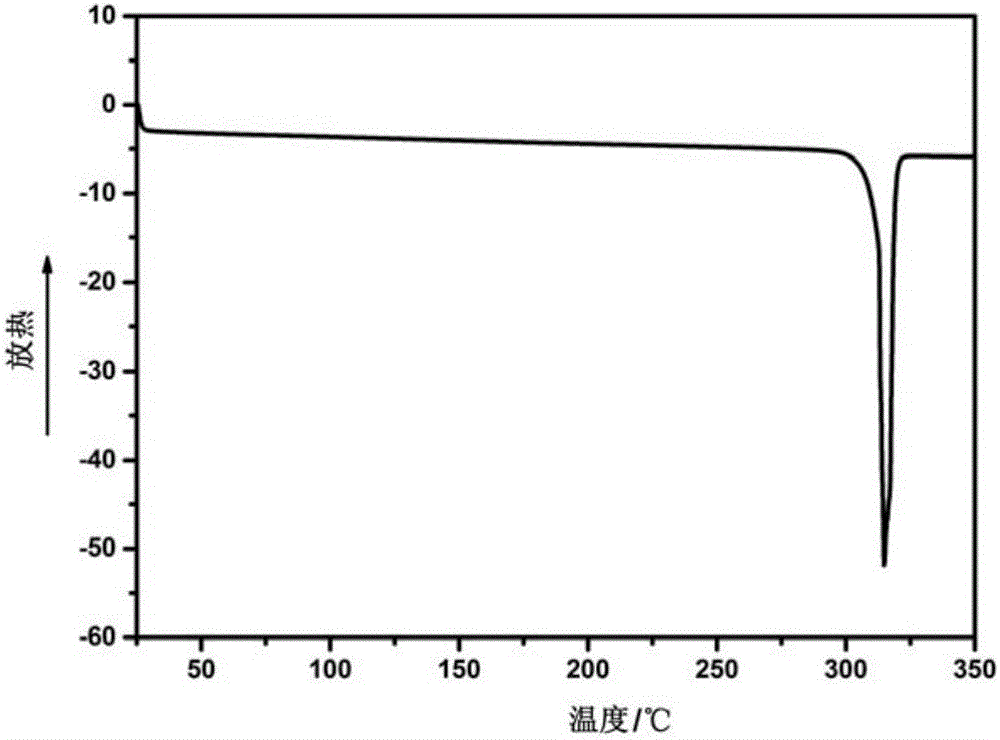
[0057] MHCF@600 is formed by the polymerization of dinitrile monomers shown in formula II. Each dinitrile monomer contains two cyano groups, and each cyano group can interact with one of the other two dinitrile monomers. The cyano group is randomly tricyclized to form a s-triazine structure, and the cyano groups between multiple dinitrile monomers undergo a tricyclic reaction, thereby connecting to form a network polymer containing a s-triazine structure, which has the following formula I Repeating structural unit:
[0058]
[0059] among them: for
[0060] for
[0061] for
[0062] Same or different
[0063] R 1 , R 2 , R 3 , R 4 Independently one of H, F, Cl, Br, I, COOH, alkoxy;
[0064] R 1 , R 2 , R 3 , R 4 Same or different
[0065] Here is a list of one of the typical structures contained in network polymers, as shown below:
[0066]
[0067] In this embodiment, R 1 , R 2 ,...
Embodiment 2
[0095] In this example, MHCF@650 was prepared through coupling reaction and polymerization reaction.
[0096] (1) Preparation of PDBN
[0097] As described in Example 1.
[0098] (2) Preparation of MHCF@650
[0099] Except that the polymerization temperature in step (2) of Example 1 is changed from 600°C to 650°C, other processes and parameters are the same as in Example 1. The yield of the obtained polymer MHCF@650 was 320 mg, and the yield was 64%.
[0100] The nitrogen absorption and desorption curve and pore size distribution of MHCF@650 are shown in Image 6 .
[0101] In order to further verify its electrical properties, electrochemical performance tests were carried out. The test method is as described in Example 1.
[0102] The cyclic voltammetry curve, constant current charge and discharge curve, change of specific capacity with current density and AC impedance spectrum of electrode materials made by MHCF@650 Figure 8-11 .
[0103] After the electrode material polymerized at 6...
Embodiment 3
[0105] In this example, MHCF@700 was prepared through coupling reaction and polymerization reaction.
[0106] (1) Preparation of PDBN
[0107] As described in Example 1.
[0108] (2) Preparation of MHCF@700
[0109] Except that the polymerization temperature in step (2) of Example 1 was changed from 600°C to 700°C, the other processes and parameters were the same as in Example 1. The yield of the obtained polymer MHCF@650 was 360 mg, and the yield was 72%.
[0110] Refer to the nitrogen adsorption and desorption curve and pore size distribution of MHCF@700 Image 6 .
[0111] In order to further verify its electrical properties, electrochemical performance tests were carried out. The test method is as described in Example 1.
[0112] The cyclic voltammetry curve, constant current charge and discharge curve, change of specific capacity with current density and AC impedance spectrum of the electrode material prepared by MHCF@700 are shown in the attached Figure 8-11 .
[0113] After the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Specific capacitance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com