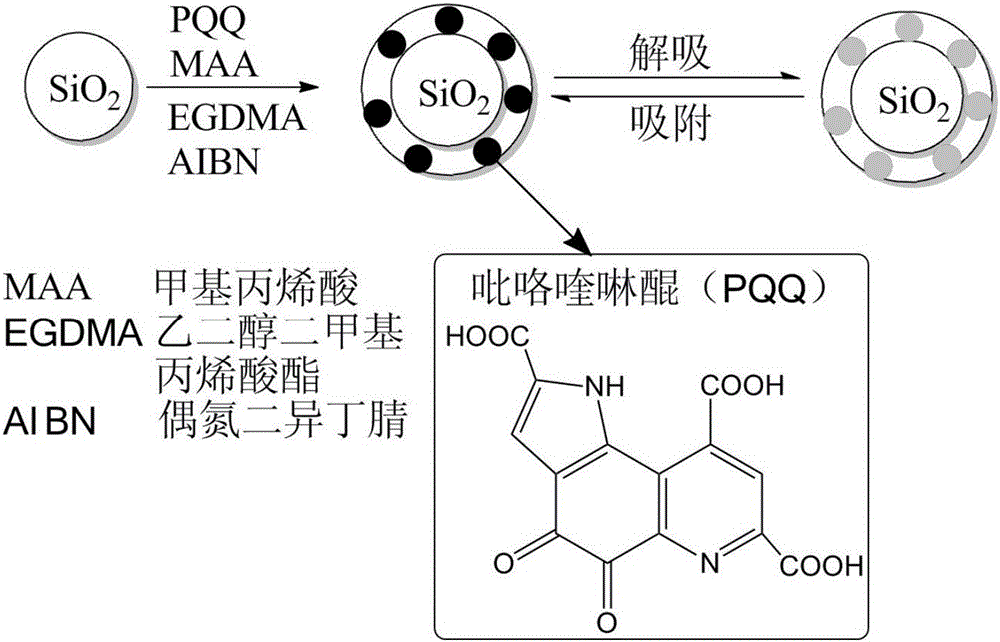
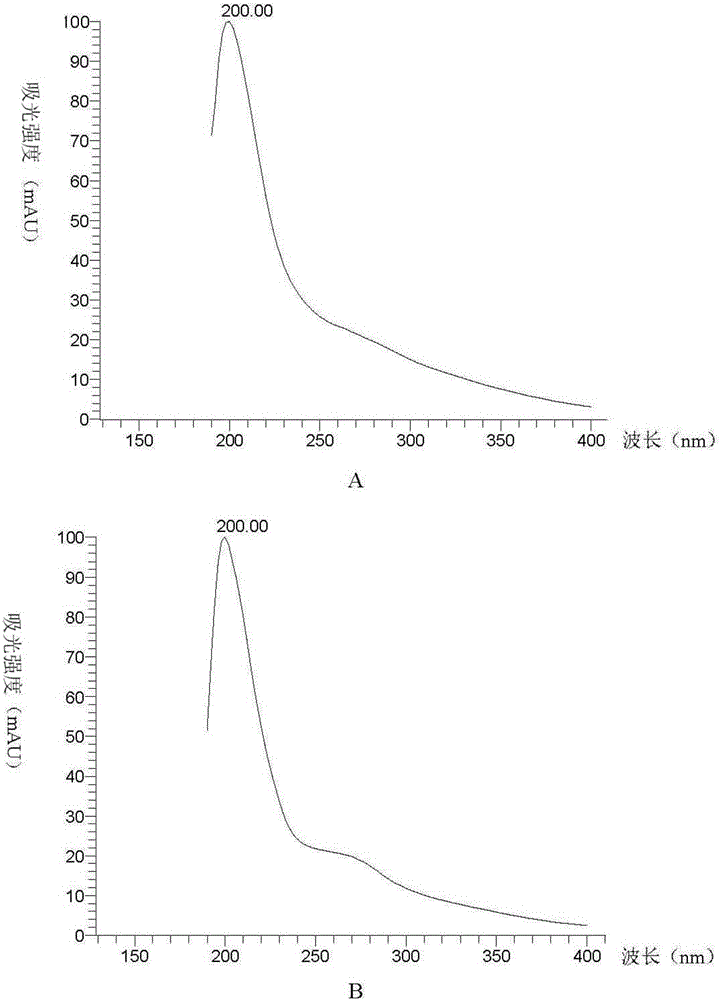
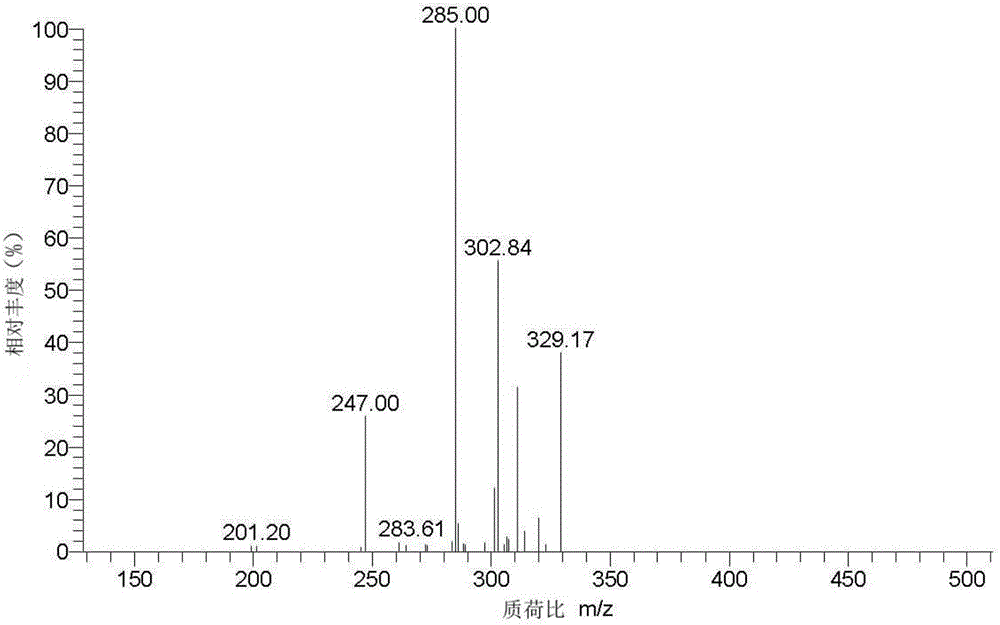
Method for separating and purifying pyrroloquinoline quinone in fermentation liquid by molecularly imprinted solid-phase extraction method
A pyrroloquinoline quinone, solid-phase extraction technology, applied in chemical instruments and methods, organic chemistry, other chemical processes and other directions, to achieve the effect of promoting industrialization development, simple process operation, and convenient large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A method for separating and purifying pyrroloquinoline quinone in fermentation broth by molecular imprinted solid phase extraction, comprising the following steps:
[0041](1) Preparation: Utilize pyrroloquinoline quinone producing bacteria to prepare a fermentation broth containing pyrroloquinoline quinone. The specific method is: inoculate Gluconobacter oxydans in the fermentation medium according to the inoculation amount of 5%, and inoculate the fermentation medium at 28° C. Shake culture for 3 days to obtain seed liquid; then inoculate the seed liquid into enriched medium according to the inoculum amount of 10%, shake culture at 28°C for 5 days, centrifuge the culture liquid at 5°C and 9000r / min for 15min, and collect the supernatant to obtain a fermented liquid rich in pyrroloquinoline quinone whose pyrroloquinoline quinone concentration is 100-200mg / L;
[0042] (2) Extraction and separation: the fermentation broth containing pyrroloquinoline quinone is extracted ...
Embodiment 2
[0048] A method for separating and purifying pyrroloquinoline quinone in fermentation broth by molecular imprinted solid phase extraction, comprising the following steps:
[0049] (1) Preparation: Utilize pyrroloquinoline quinone producing bacteria to prepare a fermentation broth containing pyrroloquinoline quinone. The specific method is: inoculate Gluconobacter oxydans in the fermentation medium according to the inoculation amount of 5%, and inoculate the fermentation medium at 28° C. Shake culture for 3 days to obtain seed liquid; then inoculate the seed liquid into enriched medium according to the inoculum amount of 10%, shake culture at 28°C for 5 days, centrifuge the culture liquid at 5°C and 9000r / min for 15min, and collect the supernatant to obtain a fermented liquid rich in pyrroloquinoline quinone whose pyrroloquinoline quinone concentration is 100-200mg / L;
[0050] (2) Extraction and separation: the fermentation broth containing pyrroloquinoline quinone is extracted...
Embodiment 3
[0056] A method for separating and purifying pyrroloquinoline quinone in fermentation broth by molecular imprinted solid phase extraction, comprising the following steps:
[0057] (1) Preparation: Utilize pyrroloquinoline quinone producing bacteria to prepare a fermentation broth containing pyrroloquinoline quinone. The specific method is: inoculate Gluconobacter oxydans in the fermentation medium according to the inoculation amount of 5%, and inoculate the fermentation medium at 28° C. Shake culture for 3 days to obtain seed liquid; then inoculate the seed liquid into enriched medium according to the inoculum amount of 10%, shake culture at 28°C for 5 days, centrifuge the culture liquid at 5°C and 9000r / min for 15min, and collect the supernatant to obtain a fermented liquid rich in pyrroloquinoline quinone whose pyrroloquinoline quinone concentration is 100-200mg / L;
[0058] (2) Extraction and separation: the fermentation broth containing pyrroloquinoline quinone is extracted...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com