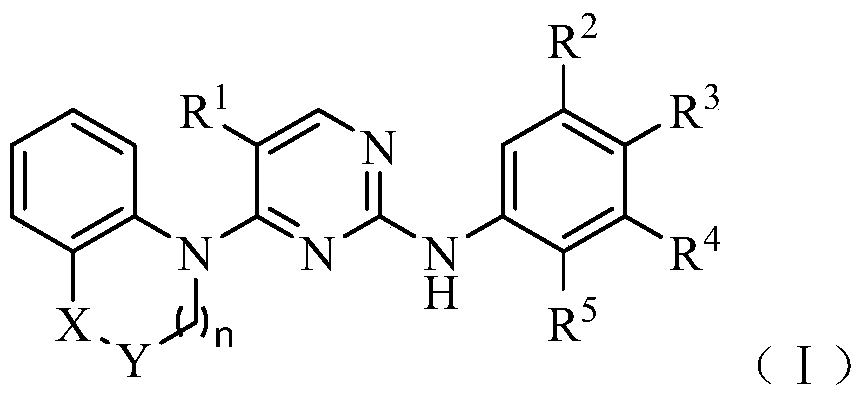
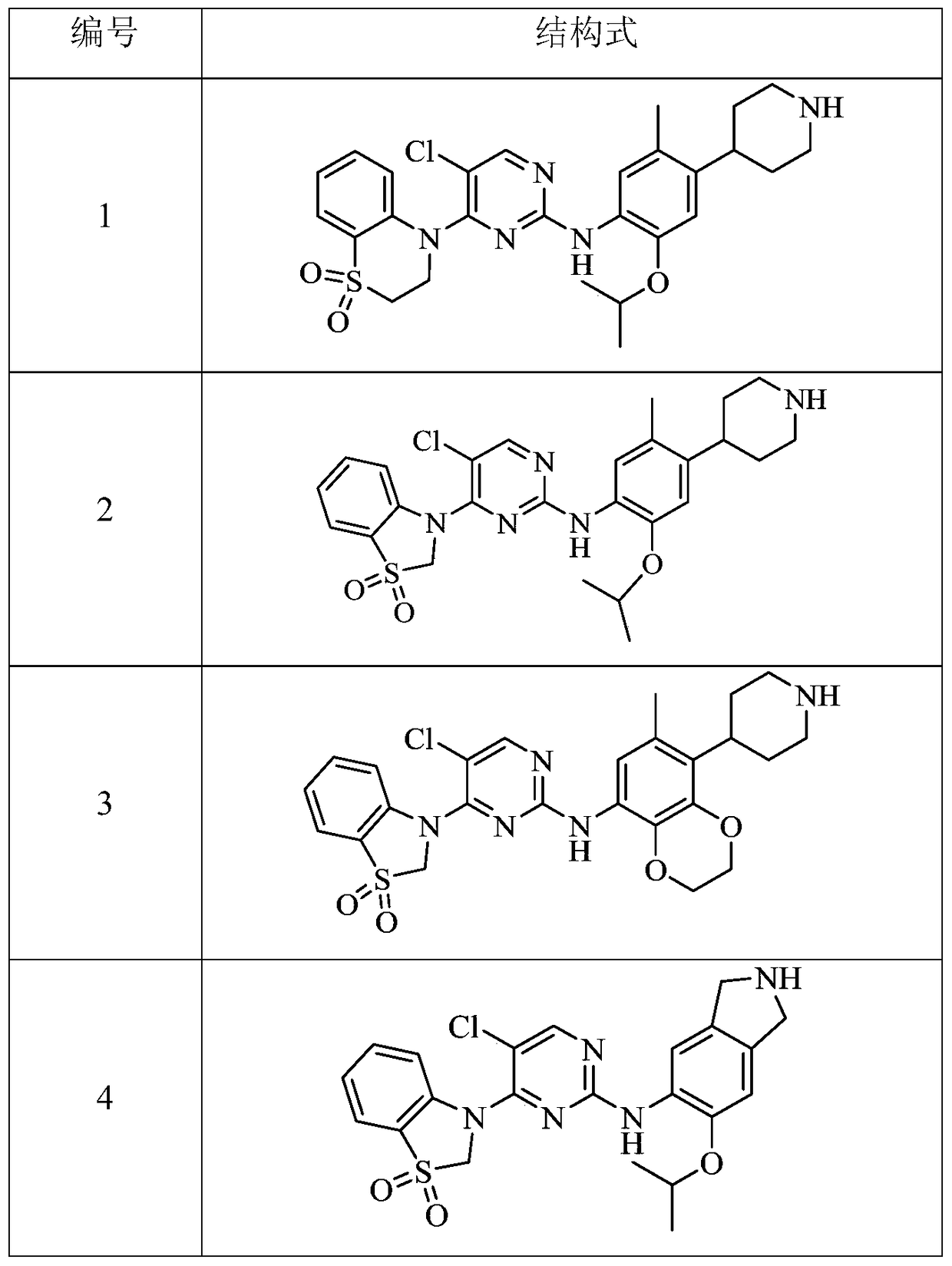
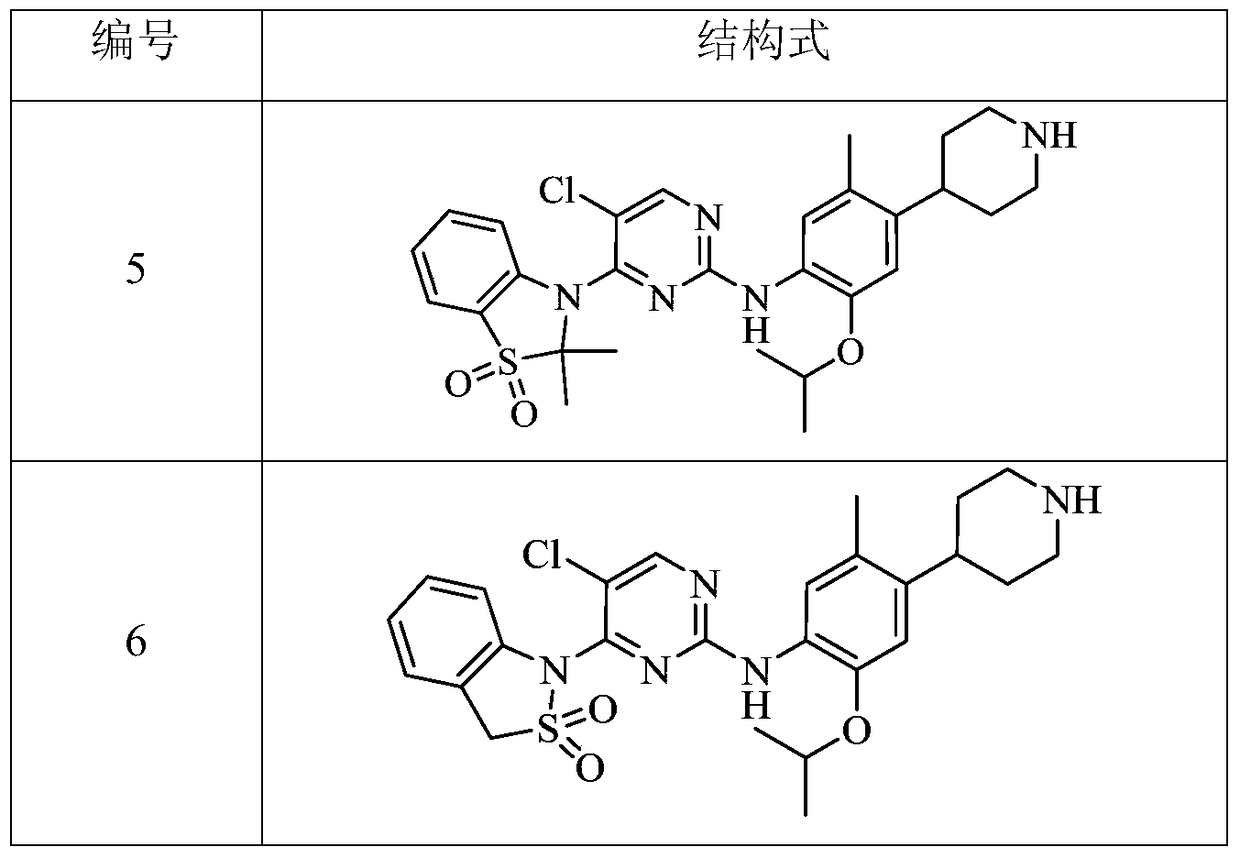
bicyclic anaplastic lymphoma kinase inhibitor
A kind of technology of heterocyclic group and alkyl group, applied in the field of bi-cyclic anaplastic lymphoma kinase inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0142] The preparation of step 1 intermediate 1
[0143] Intermediate 1 was purchased or prepared separately.
[0144] Step 2 Preparation of Intermediate 2
[0145] Intermediate 1 was dissolved in a suitable solvent, sodium hydride was added at 0°C, stirred, and then raw material 1 was added, the reaction mixture was stirred at room temperature (for example, 1-12h), quenched by adding water, extracted with an organic solvent (for example, ethyl acetate), concentrated, and purified by Purification (eg silica gel column chromatography) affords intermediate 2.
[0146] Step 3 Preparation of the compound of general formula (I) of the present invention
[0147] Dissolve intermediate 2 and intermediate 3 in a suitable solvent (such as 1,4-dioxane), under nitrogen protection, heat (such as 70°C-120°C) for reaction (such as 4-16 hours), filter, and add water Dilute, extract with an organic solvent (such as ethyl acetate), dry, concentrate, and obtain the compound of general formula...
experiment example 1
[0175] Experimental Example 1 The in vitro enzymatic activity test of the compound of the present invention
[0176] Test product: trifluoroacetate salt of compound 2, compound 3 and compound 4 of the present invention, prepared according to the preparation examples of trifluoroacetate salt of compound 2, compound 3 and compound 4.
[0177] The meanings represented by the abbreviations of the following experiments are as follows:
[0178] DMSO: dimethyl sulfoxide
[0179] DTT: Dithiothreitol
[0180] SEB: Enzyme Catalyst Buffer
[0181] ATP: adenosine triphosphate
[0182] ALK: Anaplastic Lymphoma Kinase
[0183] SA-XL665: Streptavidin-labeled donor
[0184] 2.5×, 5×, 10× “×” among them: times
[0185] experimental method:
[0186] ALK kinase buffer preparation:
[0187] Take an appropriate amount of MgCl with a mother liquor concentration of 1000mM 2 , 2500nM SEB, 100mM DTT, and 5×enzyme buffer were added to ultrapure water so that the final concentrations were: 5mM...
Embodiment 1
[0208] Example 1 4-(5-chloro-2-((2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)amino)pyrimidine-4- base)-3,4-dihydro-2H-benzo[b][1,4]thiazine 1,1-dioxide (compound 1) trifluoroacetate
[0209]
[0210] (1) Preparation of 2-((2-chloroethyl)thio)aniline
[0211]
[0212] In a 250mL three-neck flask, add 2-aminothiophenol (10g, 79.88mmol) and 1,2-dichloroethane (78g, 788.3mmol). The reaction mixture was heated to reflux under the protection of nitrogen, and then a methanol solution of sodium methoxide (15 g) was added dropwise. After the addition was complete, it was heated to reflux for 3 hours. After the reaction was completed, the reaction solution was cooled and poured into ice water, then extracted with dichloromethane (3×100mL), the organic layers were combined, washed with saturated sodium bicarbonate, the organic layer was separated, dried with anhydrous sodium sulfate, and concentrated to obtain the product (8 g, 53% yield).
[0213] (2) Preparation of N-(2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com