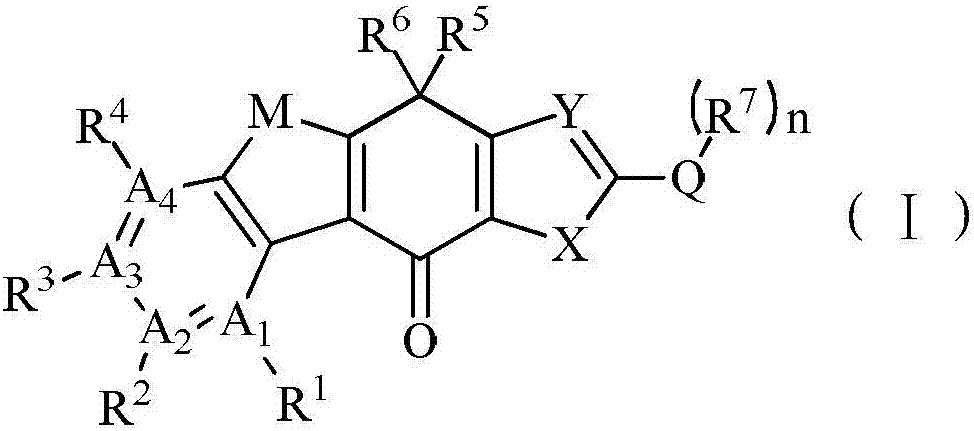
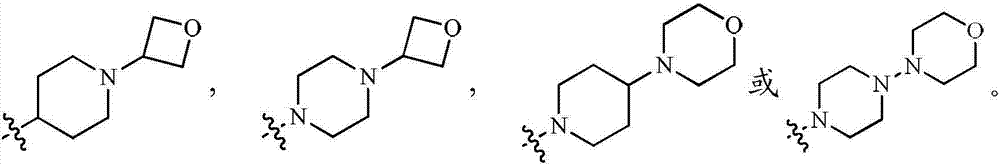
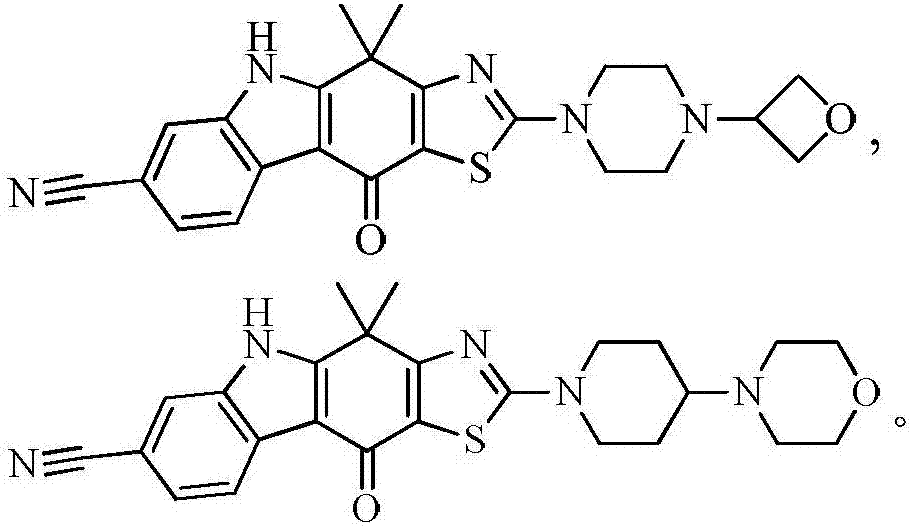
tetracycline kinase inhibitors
An immunosuppressant, selected technology, applied in the field of medical technology, and can solve problems such as easy generation of drug resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0121] Step 1 Preparation of intermediate 1
[0122] Dissolve raw material 1 in a solvent (such as glacial acetic acid), add hydrobromic acid solution (such as 40% hydrobromic acid solution), cool in an ice-water bath, add an appropriate amount of bromine-dissolved solvent (such as glacial acetic acid) solution dropwise, Stir until the raw materials are completely consumed, cool (for example, add ice water), extract (with ethyl acetate), and separate and purify the combined organic phases (for example, by silica gel column chromatography) to obtain intermediate 1.
[0123] Step 2 Preparation of intermediate 3
[0124] Intermediate 1 and Intermediate 2 (for example, 1.2 equivalents) were dissolved in a solvent (such as tetrahydrofuran), heated to reflux until the reaction was complete, the solvent was removed by rotary evaporation, and intermediate 3 was obtained by separation and purification (for example, by silica gel column chromatography).
[0125] Step 3 Preparation of I...
experiment example 1
[0154] Experimental example 1 In vitro enzymatic activity test of the compound of the present invention
[0155] Test articles: compound 1 and compound 2 of the present invention, see the preparation examples of compound 1 and compound 2 for their chemical names and preparation methods.
[0156] Control drug: CH5424802, self-made (prepared with reference to the preparation method of patent CN102459172A)
[0157] The meanings represented by the abbreviations of the following experiments are as follows:
[0158] DMSO: dimethyl sulfoxide
[0159] DTT: Dithiothreitol
[0160] SEB: Enzyme Catalyst Buffer
[0161] ATP: adenosine triphosphate
[0162] ALK: Anaplastic Lymphoma Kinase
[0163] SA-XL665: Streptavidin-labeled donor
[0164] 2.5×, 5×, 10× “×” among them: times
[0165] experimental method:
[0166] ALK kinase buffer preparation:
[0167] Take 20 μL of MgCl2 with a mother solution concentration of 1000 mM, 40 μL of SEB with a mother solution concentration of 2500 ...
experiment example 2
[0187] Experimental example 2 In vitro cell activity test of the compound of the present invention
[0188] Test articles: compound 1 and compound 2 of the present invention, see the preparation examples of compound 1 and compound 2 for their chemical names and preparation methods.
[0189] The control drug CH5424802 is self-made (prepared with reference to the preparation method of patent CN102459172A), and its structural formula is as described in the background technology.
[0190] The meanings represented by the abbreviations of the following experiments are as follows:
[0191] rpm: revolutions per minute
[0192] DMSO: dimethyl sulfoxide
[0193] MTS: Thiazolium blue tetrazolium bromide
[0194] RPMI1640: 1640 medium (RPMI: Roswell Park Memorial Institute)
[0195] "×" in 500×, 1000×, 10×: times
[0196] experimental method:
[0197] (1) NCI-H3122, Karpas-299, SH-SY5Y cells:
[0198] (1) Medium preparation:
[0199] Take 10 mL of fetal bovine serum, 1 mL of a mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com