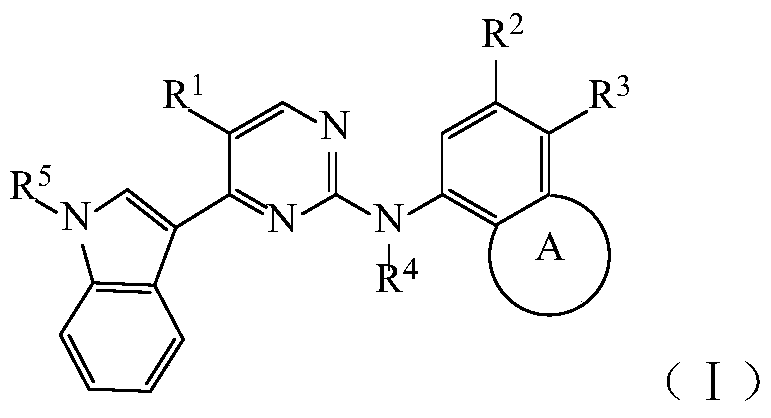
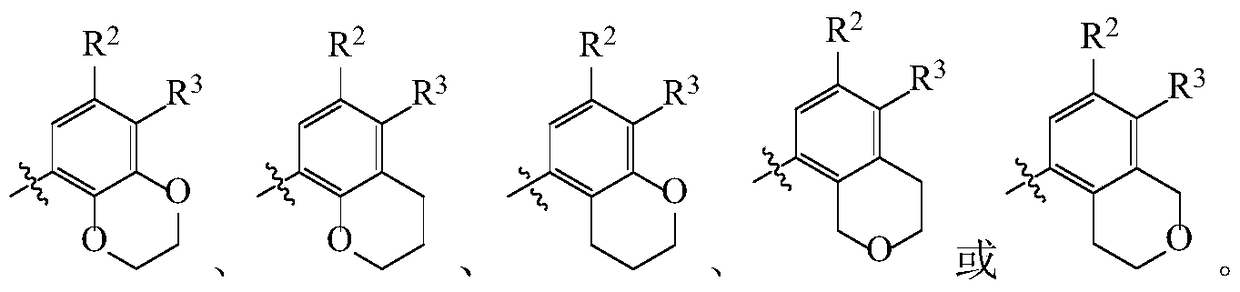
Pyrimidine Derivatives Anaplastic Lymphoma Kinase Inhibitors
A technology for immunosuppressants and drugs, applied to salts, esters or solvates of receptors, which can solve problems such as easy generation of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] For the preparation method of intermediate 8, refer to patent 201410072908.3.
[0075] The preparation of step 1 intermediate 1
[0076] Intermediate 1 was purchased or prepared separately.
[0077] Step 2 Preparation of intermediate 2
[0078] Intermediate 1 is dissolved in an appropriate solvent (such as N,N-dimethylformamide), and an appropriate amount (such as 2 equivalents) of N-bromosuccinimide is added, heated (such as 55°C) and stirred overnight, cooled to room temperature, and added with water The reaction is quenched, extracted with an organic solvent (such as ethyl acetate), concentrated, and purified by an appropriate method (such as silica gel column chromatography) to obtain intermediate 2.
[0079] Step 3 Preparation of intermediate 3
[0080] Dissolve intermediate 2 in a suitable solvent (e.g. ethanol), add acetic acid, add reduced iron powder in batches (e.g. at 70°C), heat (e.g. 80°C) and stir (e.g. 3 hours), filter off the solid, and add water to q...
experiment example 1
[0111] Experimental Example 1 The in vitro enzymatic activity test of the compound of the present invention
[0112] Test product: trifluoroacetate of compound 1 of the present invention, its chemical name and preparation method are shown in the preparation example of compound 1 trifluoroacetate.
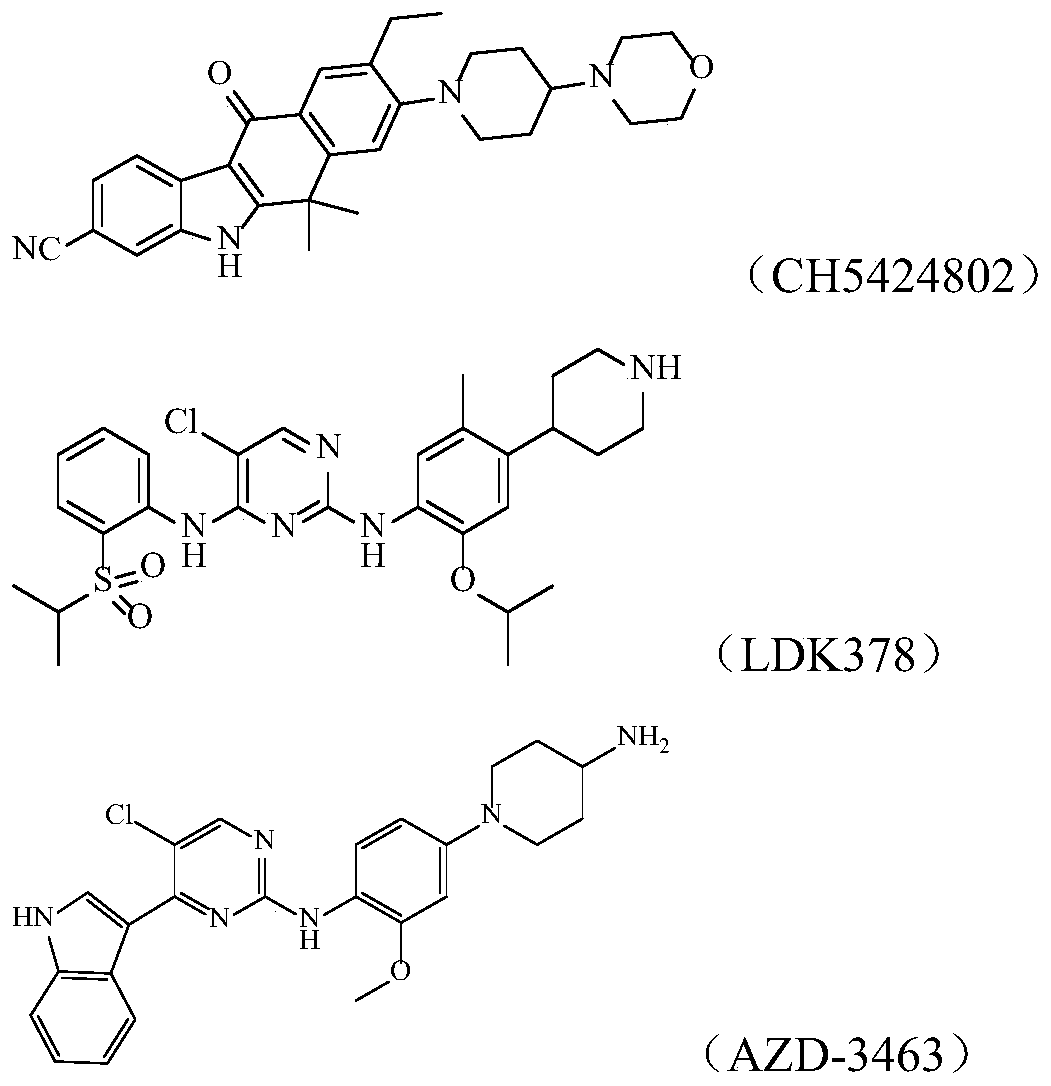
[0113] Control drug: LDK378, self-made (prepared with reference to the preparation method of patent WO2008 / 073687A2).
[0114] The meanings represented by the abbreviations of the following experiments are as follows:
[0115] DMSO: dimethyl sulfoxide
[0116] DTT: Dithiothreitol
[0117] SEB: Enzyme Catalyst Buffer
[0118] ATP: adenosine triphosphate
[0119] ALK: Anaplastic Lymphoma Kinase
[0120] SA-XL665: Streptavidin-labeled donor
[0121] 2.5×, 5×, 10× “×” among them: times
[0122] experimental method:
[0123] ALK kinase buffer preparation:
[0124] Take 20 μL of MgCl2 with a mother solution concentration of 1000 mM, 40 μL of SEB with a mother solution concentrat...
experiment example 2
[0143] Experimental Example 2 The in vitro cell activity test of the compound of the present invention
[0144] Test product: trifluoroacetate salt of compound 1 of the present invention, see the preparation example of trifluoroacetate salt of compound 1 for its chemical name and preparation method.
[0145] The control drug 1LDK-378 is self-made (refer to the preparation method of patent WO2008 / 073687A2), and its structural formula is as described in the background technology.
[0146] The reference drug 2CH5424802 is self-made (prepared with reference to the preparation method of patent CN102459172A), and its structural formula is as described in the background technology.
[0147] The meanings represented by the abbreviations of the following experiments are as follows:
[0148] rpm: revolutions per minute
[0149] DMSO: dimethyl sulfoxide
[0150] MTS: Thiazolium blue tetrazolium bromide
[0151] RPMI1640: 1640 medium (RPMI: Roswell Park Memorial Institute)
[0152]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com