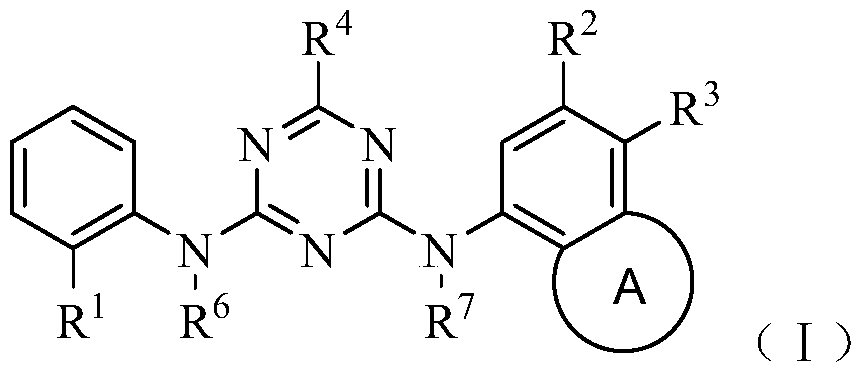
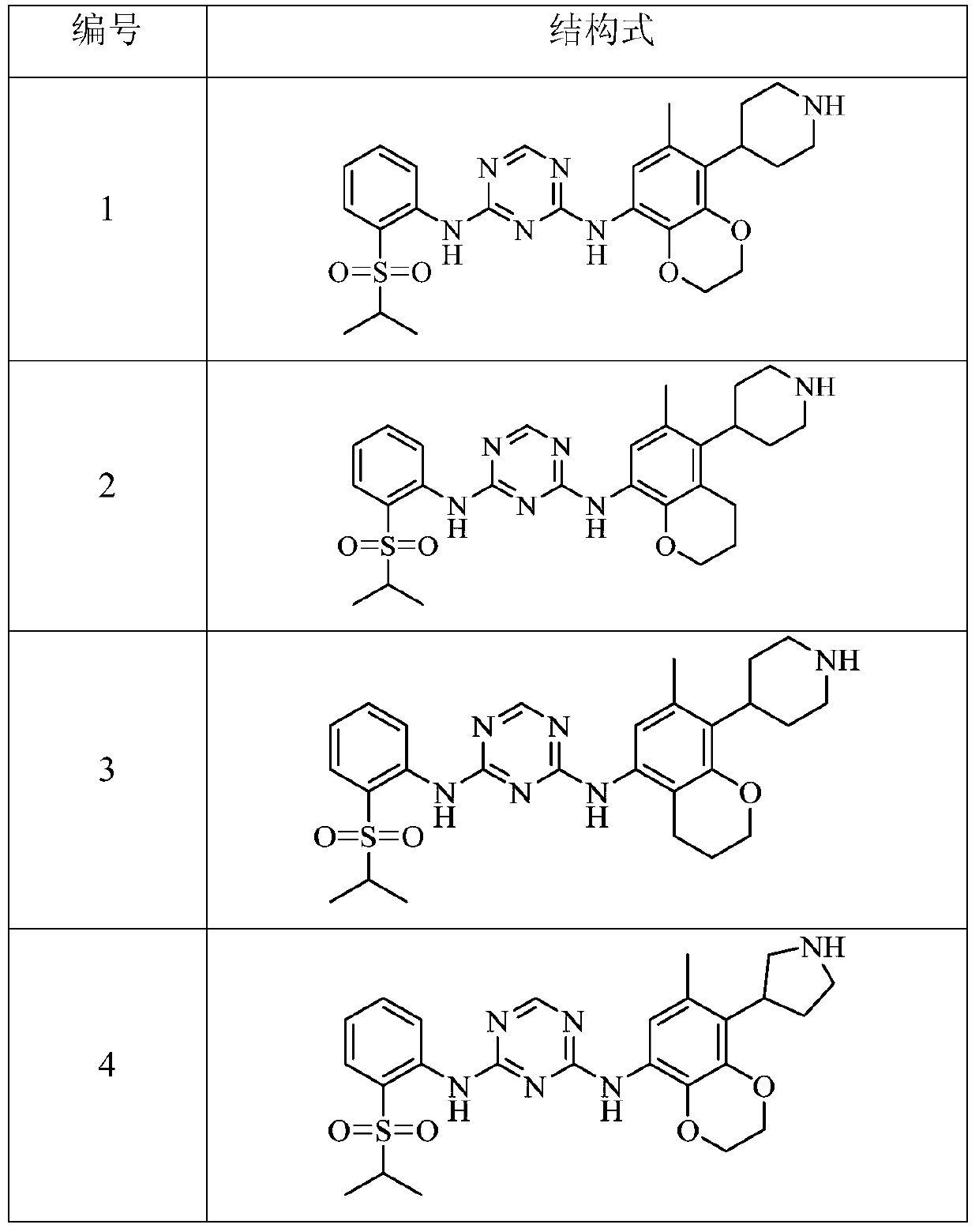
Triazine Derivatives Anaplastic Lymphoma Kinase Inhibitors
An inhibitor, lymphoma technology, applied in the field of triazine derivative anaplastic lymphoma kinase inhibitors, which can solve problems such as easy generation of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
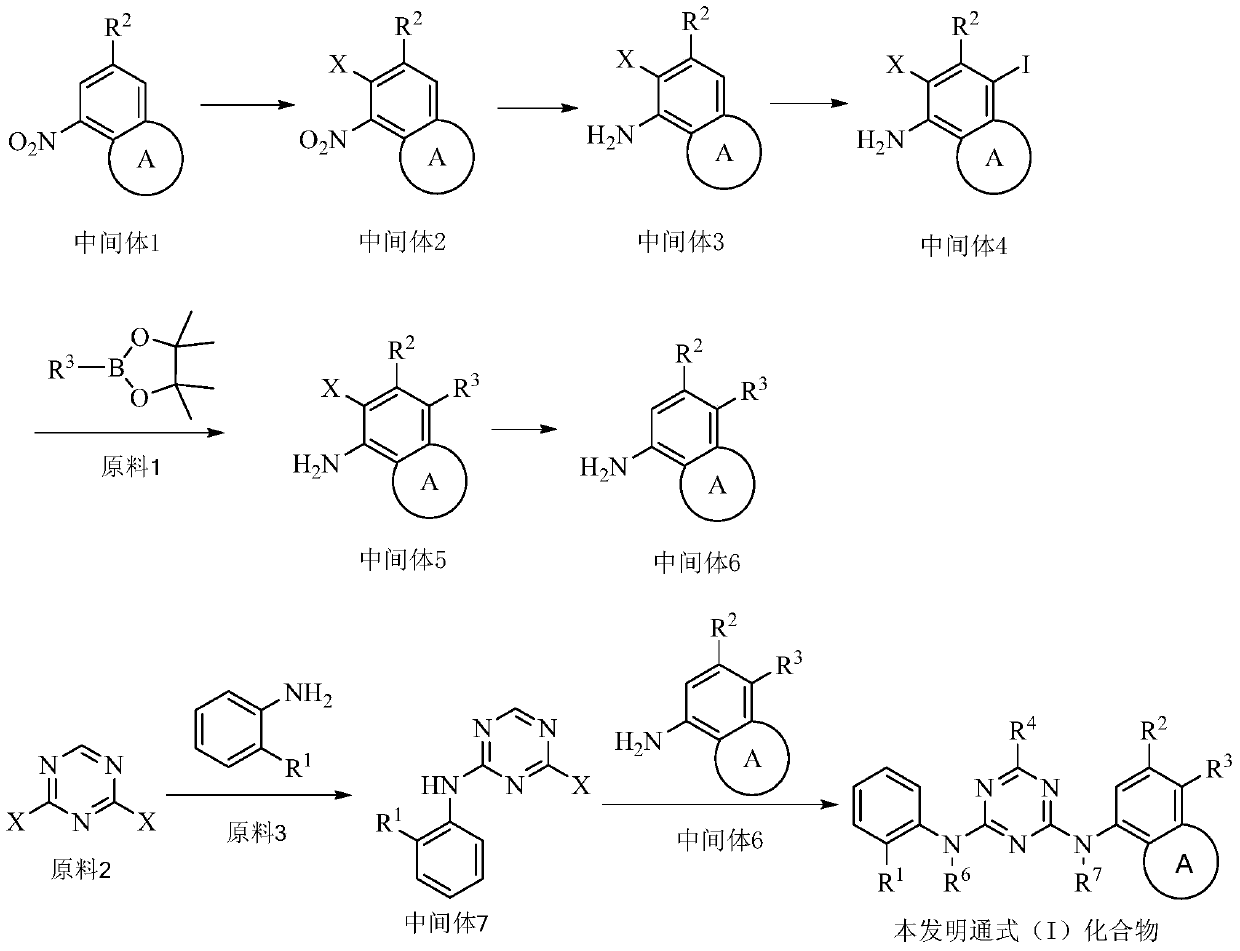
[0088] The present invention also provides the preparation method of above-mentioned compound, but not limited to following method, and reaction equation is as follows:
[0089]
[0090] Reaction steps:
[0091] The preparation of step 1 intermediate 1
[0092] Intermediate 1 can be purchased or prepared by appropriate methods.
[0093] Step 2 Preparation of Intermediate 2
[0094] Intermediate 1 is dissolved in a solvent (such as N,N-dimethylformamide), added N-bromosuccinimide, heated (such as 50-70°C) overnight, the reaction is complete, quenched with water, organic solvent (eg ethyl acetate) extraction, concentration and purification (eg silica gel column chromatography) to obtain intermediate 2.
[0095] Step 3 Preparation of Intermediate 3
[0096] Dissolve intermediate 2 in a suitable solvent (such as ethanol), add an organic solvent (such as acetic acid), heat (such as 70°C), add iron powder, heat up (such as 80°C), react (such as 2-4 hours), and react Complete...
experiment example 1
[0125] Experimental Example 1 The in vitro enzymatic activity test of the compound of the present invention
[0126] Test product: trifluoroacetate salt of compound 1 of the present invention, its chemical name and preparation method are shown in the preparation example of trifluoroacetate salt of compound 1.
[0127] The meanings represented by the abbreviations of the following experiments are as follows:
[0128] DMSO: dimethyl sulfoxide
[0129] DTT: Dithiothreitol
[0130] SEB: Enzyme Catalyst Buffer
[0131] ATP: adenosine triphosphate
[0132] ALK: Anaplastic Lymphoma Kinase
[0133] SA-XL665: Streptavidin-labeled donor
[0134] 2.5×, 5×, 10× “×” among them: times
[0135] experimental method:
[0136] ALK kinase buffer preparation:
[0137] Take an appropriate amount of MgCl with a mother liquor concentration of 1000mM 2 , 2500nM SEB, 100mM DTT, and 5×enzyme buffer were added to ultrapure water so that the final concentrations were: 5mM, 25nM, 1mM, and 1×enzy...
specific Embodiment approach
[0155] The above-mentioned content of the present invention will be further described in detail through specific implementation in the form of examples below. However, it should not be construed that the scope of the above-mentioned subject matter of the present invention is limited to the following examples. All technologies realized based on the above contents of the present invention belong to the scope of the present invention.
[0156] The definitions represented by the following abbreviations are as follows:
[0157] DMF: N,N-Dimethylformamide
[0158] Pd(dppf)Cl 2 : [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride
[0159] DIPEA: Diisopropylethylamine
[0160] TFA: Trifluoroacetic acid
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com