3,5-dinitro-2,6-bis(4,4'-sulfonic azobenzene azo amino)pyridine as well as preparation method and application
A technology of sulfoazobenzene azoamino and sulfoazobenzene, which is applied in the field of bistriazene compounds, can solve the problems of low sensitivity and unsatisfactory selectivity of heavy metal ions, and achieve low cost and convenient The effect of mild storage and preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
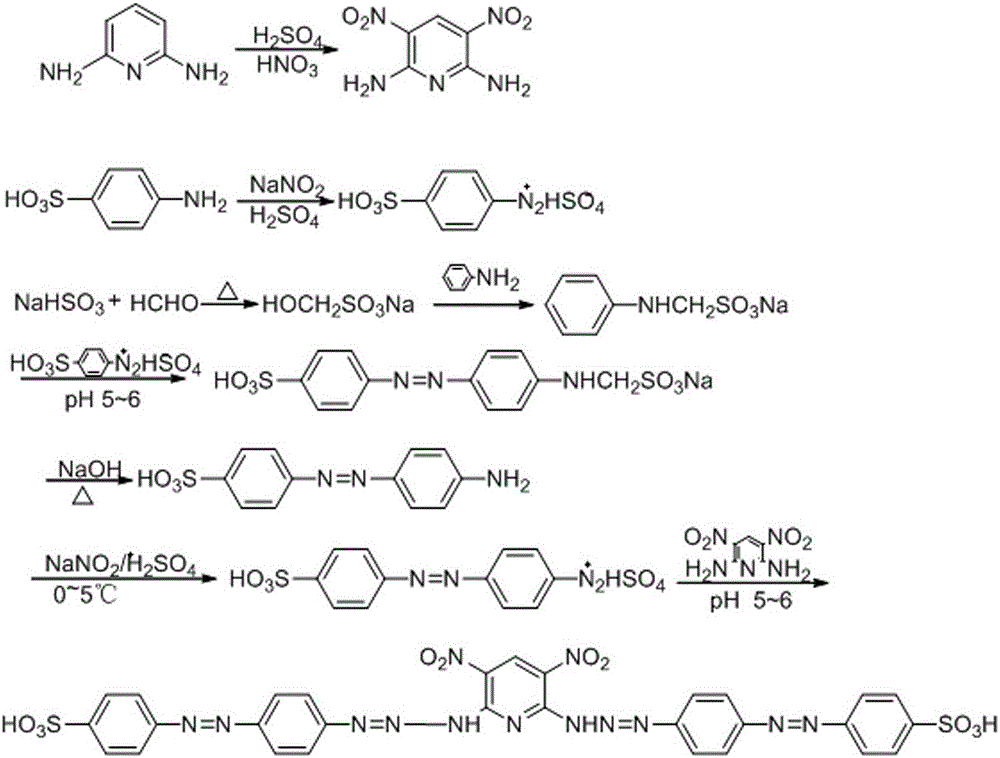
[0029] A kind of 3,5-dinitro-2,6-bis(4,4´-sulfoazophenylazoamino)pyridine (DNDSZBZAB for short), its molecular structure formula is:
[0030] .
[0031] The preparation method of 3,5-dinitro-2,6-bis(4,4'-sulfonic acid azophenyl azoamino)pyridine comprises the following steps, such as figure 1 Shown:
[0032] (1) Preparation of 4-amino-4´-sulfoazobenzene
[0033] In a beaker, add 10.4 g (0.1 mol) NaHSO3 , then add 38 mL of water and 8 mL of 40% (0.1 mol) formaldehyde by volume fraction, stir in a water bath at 60-66°C for 40 minutes, add 7.2 g (0.08 mol) of aniline, and react for 2 hours to obtain light yellow anilinomethylsulfonic acid Sodium clarified solution; another 13.8g (0.08 mol) p-aminobenzenesulfonic acid was dissolved in 140 mL of 3% sodium carbonate solution, and under ice water cooling, 5.6 g of sodium nitrite was dissolved in 30 mL of water. solution; under stirring, add 210mL of concentrated hydrochloric acid, the dropwise addition process is controlled with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com