Baohuoside-I preparation method
A technology of baohuoside and epimedium extract, applied in the field of preparation of baohuoside I, can solve the problems of unsuitable industrial production, low yield, waste of medicinal materials, etc., and achieves easy industrialized large-scale production, good selectivity, high yield high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Preparation of baohuoside Ⅰ by enzymatic hydrolysis
[0040] Take 10g of Epimedium extract containing 90% icariin, dissolve it in 140mL pH=5.9, 1 / 15M potassium dihydrogen phosphate-disodium hydrogen phosphate buffer solution, add 60mL 95% ethanol under stirring condition, add 100mL fruit Glue enzyme solution, after mixing evenly, start timing when the temperature is constant at 52-55°C, stop the reaction after 48 hours of reaction, and cool the reaction solution to 30°C.
[0041] 2. Purification of Baohuoside I
[0042] After the enzymolysis reaction, the reaction solution was centrifuged to collect the filter cake, which weighed 7.55 g after drying, added 176 g of acetone after crushing, filtered after stirring for 1 hour, added 129 g of purified water to the acetone solution, and heated to reflux until the solution was clear. Then the solution was slowly cooled to 14° C. and kept warm for 2 hours, filtered, and the filter cake was collected and dried to obtain...
Embodiment 2
[0044] 1. Preparation of baohuoside Ⅰ by enzymatic hydrolysis
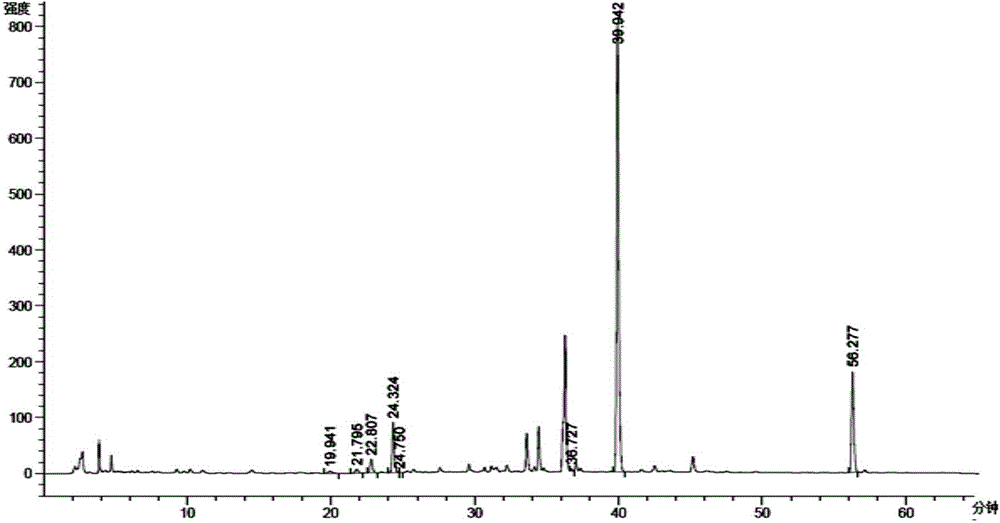
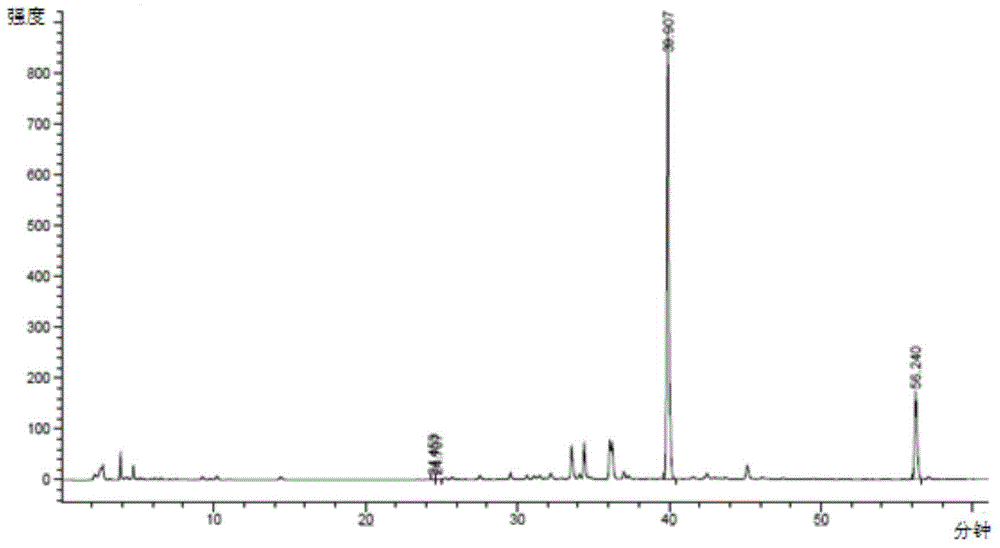
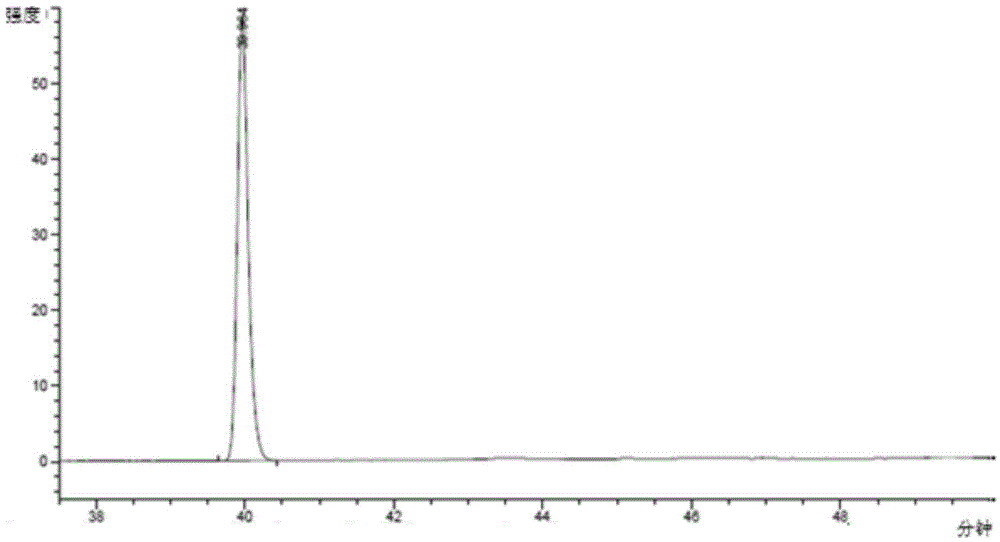
[0045] Take 3.75kg of Epimedium extract containing 94% icariin, dissolve it in 52L pH=5.9, 1 / 15M potassium dihydrogen phosphate-disodium hydrogen phosphate buffer solution, add 24L 95% ethanol under stirring condition, 75L Pectinase solution, after mixing evenly, start timing when the temperature is constant at 52-55°C, stop the reaction after reacting for 48 hours, and cool the reaction solution to 30°C. The high performance liquid chromatography of reacting 12 hours and 36 hours is respectively as follows figure 1 and figure 2 shown. pass figure 1 and figure 2 compare and contrast image 3 , it can be seen that with the increase of enzymatic hydrolysis time, the concentration of icariin and other substrates relative to baohuoside I decreased, indicating that with the prolongation of enzymatic hydrolysis time, icariin and other substrates were converted into baohuoside I.
[0046] 2. Purification of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com