Boron nitride catalyst for light alkane or alkylbenzene oxydehydrogenation and preparing method and application thereof
A low-carbon alkanes and oxidative dehydrogenation technology, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem that the alkane oxidative dehydrogenation process cannot realize industrial application, restricts direct dehydrogenation process, Catalyst deactivation and other problems to achieve the effect of improving surface utilization, olefin selectivity, and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
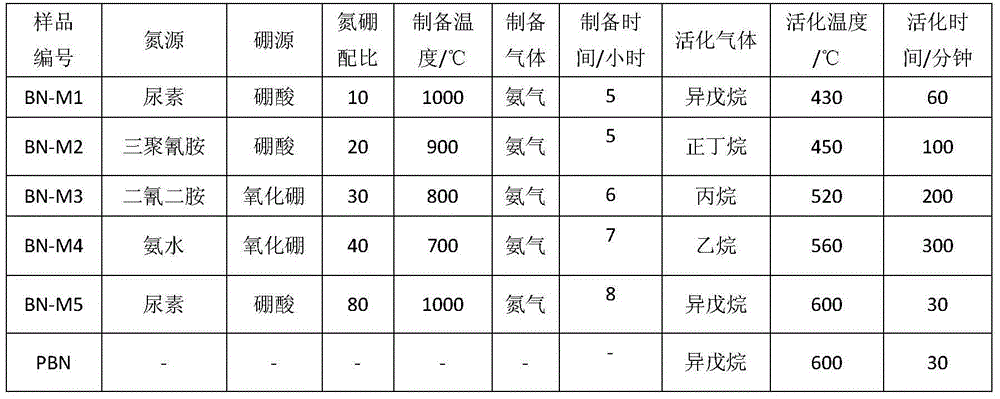
[0029] Weigh 1.24 grams of boric acid and 12.02 grams of urea. At this time, the molar ratio of nitrogen atoms to boron atoms is 10:1, add 60 ml of water, heat to 60°C and stir until completely dissolved, and evaporate all the solution in an oven at 60°C. Solvent, take out the solid, put it into a quartz boat, and pass ammonia gas into the tube furnace, and bake at 1000 ° C for 5 hours. The resulting material number is BN‐M1.
Embodiment 2~5
[0031] According to the operation steps of Example 1, the boron source, nitrogen source, boron-nitrogen atomic ratio and calcination temperature are changed non-limitatively, and the obtained boron nitride materials are sequentially numbered as BN-M2~BN-M5, and the corresponding parameters See Table 1.
Embodiment 9
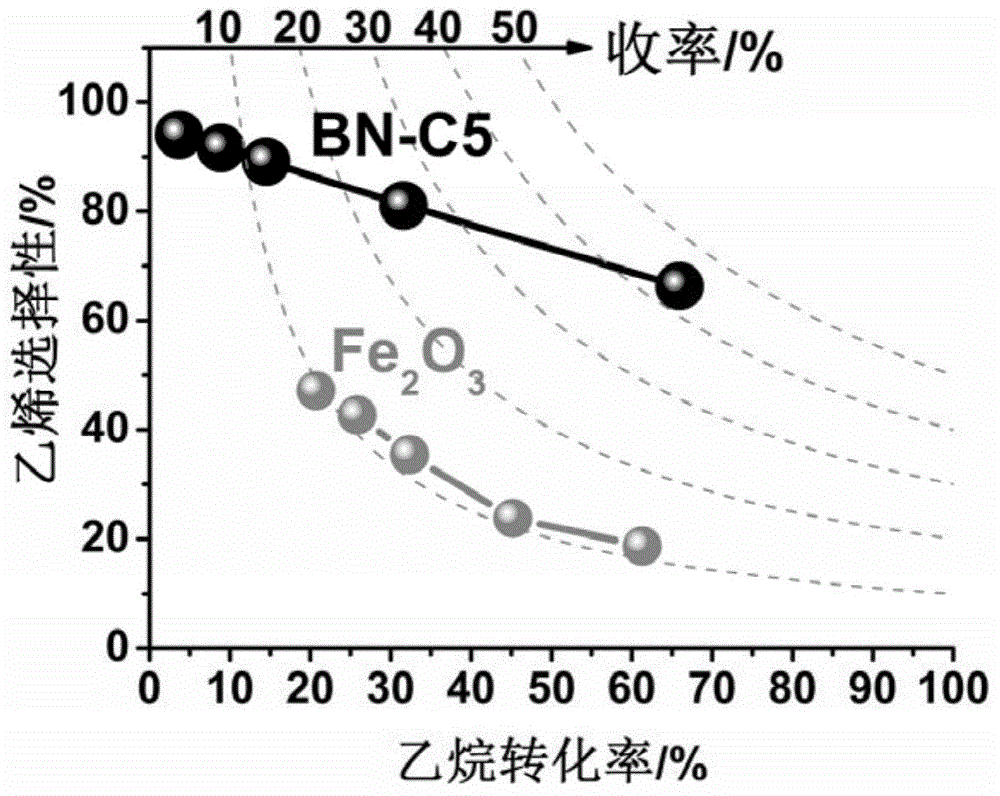
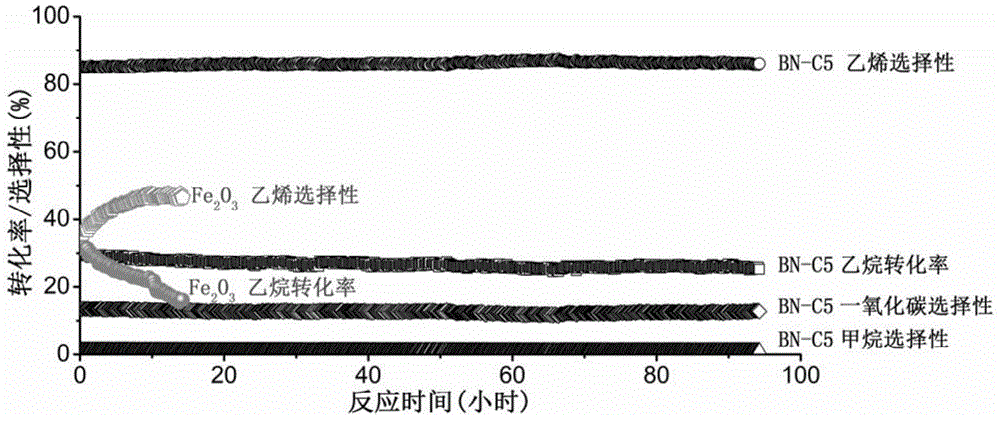
[0033]To activate the BN-M1 boron nitride material synthesized in Example 1, weigh 500 mg of BN-M1 and put it into a tube furnace, inject 50 ml / min of isopentane, and activate it at a constant temperature of 430°C for 60 minutes , the obtained catalyst number is BN-C1. Carry out catalytic performance test to BN‐C1 (results are listed in table 2): with ethane, oxygen, the mixed gas of helium gas volume ratio of 1:1:48 as reaction raw material gas, 50 milligrams of catalysts used for each test, The feed flow rate of raw material gas is 10 ml / min, the reaction temperature is 550°C, and the reaction product is detected by Agilent gas chromatograph 7890A. The method for calculating conversion and selectivity is as follows:
[0034] Alkanes conversion rate (%)=100×(moles of alkanes before reaction - moles of alkanes after reaction) / moles of alkanes before reaction
[0035] Olefin selectivity (%) = 100 x moles of total olefins produced / (moles of alkanes before reaction - moles of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com