Streptococcus-suis-disease-resistant fusion protein with autoimmune activity and preparing and application thereof
A fusion protein, Streptococcus suis technology, applied in the field of fusion proteins, can solve the problems of lack of immune adjuvant, no cross protection, no reliable preventive effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
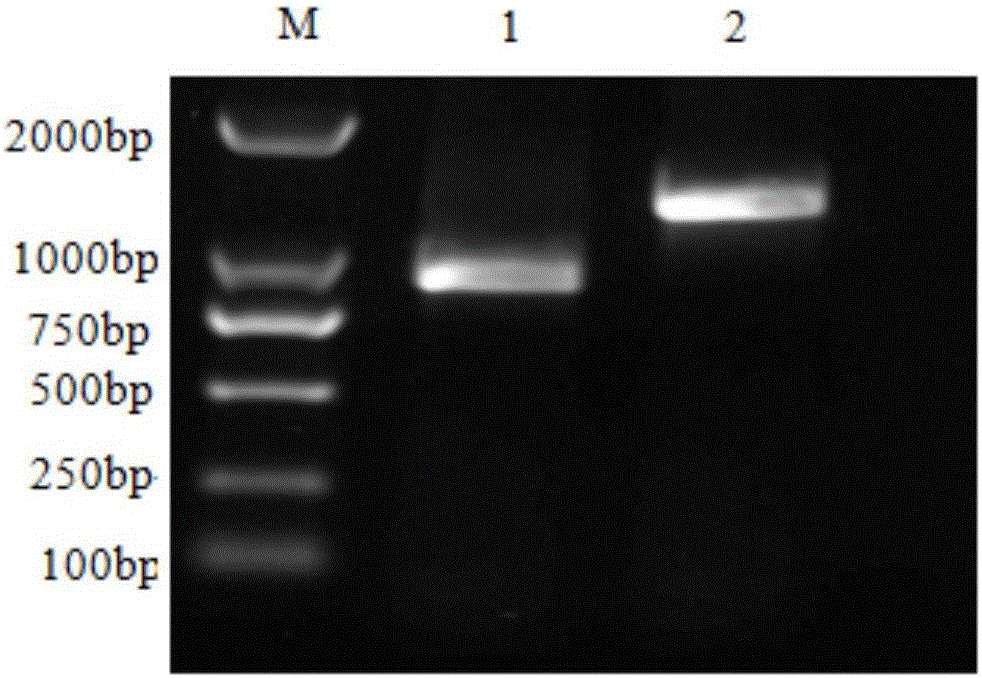
[0064] [Example 1] Sao 28‐318 and Omp16 26‐168 / Sao 28‐318 Construction of recombinant protein
[0065] 1. Design and synthesis of primers
[0066] According to published sao on GenBank 28‐318 (GenBank Gene ID: 61969363), omp16 26‐168 (GenBank Gene ID: 333601408) gene sequence, after splicing, was synthesized by Nanjing GenScript Biotechnology Co., Ltd. to obtain the following sequence Omp16 26‐168 / Sao 28‐318 , design and synthesize two pairs of primers, the primers were synthesized by Shanghai Sangon Bioengineering Technology Co., Ltd., the sequences of the primers are as follows:
[0067] 1) sao 28‐318 Gene PCR Amplification Primers
[0068] Upstream primer P1
[0069] 5'-CGCCCATGGCGTCGGCACAAGAAGTAA-3'
[0070] downstream primer P2
[0071] 5'-CGCCTCGAGCTCTTCATTAGCTATTTGC-3'
[0072] 2) Omp16 26‐168 / Sao 28‐318 Gene PCR Amplification Primers
[0073] Upstream primer P1
[0074] 5'-CGCCCATGGCGGCGTCAAAAGAAGAACCT-3'
[0075] downstream primer P2
[0076] 5'-C...
Embodiment 2
[0103] [Example 2] Identification of antigenicity of fusion protein
[0104] The fusion protein prepared above was subjected to SDS-PAGE and the gel was transferred to PVDF membrane by semi-dry method, blocked at 37°C for 1.5h, and primary antibody (the mouse serum collected after the second immunization was diluted by a certain factor) was added at 4 ℃ blocked overnight. After the membrane was washed with TBST, the secondary antibody goat anti-mouse (IgG‐HRP) diluted at 1:2000 was added, and placed on a shaker at room temperature for 2 hours. After the secondary antibody was washed away, the two primary antibodies and the fusion protein were detected with a chemiluminescent substrate. response situation. The results showed that corresponding bands appeared at the position of the size of the target protein, indicating that the fusion protein could specifically react with mouse serum and had good immunogenicity.
Embodiment 3
[0105] [Example 3] fusion protein Omp16 26‐168 / Sao 28‐318 Can induce a strong humoral immune response
[0106] Detection of antibody levels induced by fusion proteins
[0107] Detection of anti-Omp16 in serum of immunized mice by conventional ELISA 26‐168 / Sao 28‐318 Specific antibody levels. Specifically, to artificially synthesize and identify the correct Omp16 26‐168 / Sao 28‐31 8 Coat the 96-well ELISA plate, overnight at 4°C; wash the plate 3 times with PBST, add blocking solution and incubate at 37°C for 2 hours; wash 3 times with PBST, dilute the serum to be tested with PBST buffer 1:100, and incubate at 37°C 1.5h. After washing the plate 3 times, add HPR-labeled goat anti-mouse IgG-HRP and incubate at 37°C for 1 h; wash the plate 5 times with PBST, develop color with TMB in the dark, 2mol / L H 2 SO 4 The reaction was terminated, and the OD value at 450nm was read with a microplate reader to determine the serum antibody level. The results showed that the fusion...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com