Recombinant gene of glutamate dehydrogenase and its acquisition method and application
A glutamate dehydrogenase and recombinant gene technology, applied in the field of glutamate dehydrogenase recombinant gene and its acquisition, can solve the problems of unsuitability for industrial production, low enzyme yield and high cost, and achieve low production cost, High degradation efficiency and short production time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Step 1: Cell preparation:
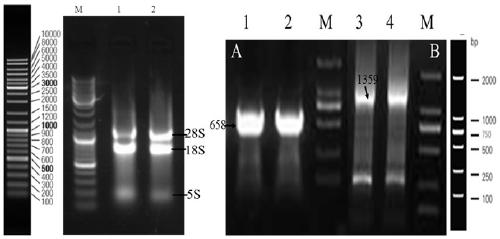
[0109] Inoculate Geotrichum candidum CCTCC NO: M 208167 strain in potato juice glucose liquid medium (20% potato juice 1000mL, glucose 20 g, natural pH), culture at 28°C, 160 rpm for 18 hours, press 2.0% The inoculum amount was inserted into the above-mentioned culture medium to expand the culture, the conditions were the same as above, and the culture time was 38h. The obtained culture was centrifuged at 5000rpm and 4°C for 10min, and the bacteria were collected, resuspended and centrifuged with sterile water, repeated 5 times, and finally Centrifuge at 8000rpm and 4°C for 10min to collect the wet cells, induce treatment with MSM medium at 28°C and 160rpm for 6h, then centrifuge at 5000rpm and 4°C for 10min to collect the cells, wash them with sterile water for 5 times, and finally Centrifuge at 8 000 r / m for 10 min at 4°C to collect the cells and extract total RNA.
[0110] Step 2: Extraction of bacterial total RNA:
[0111] The total RNA...
Embodiment 2
[0114] Step 1: Cell preparation:
[0115] With embodiment 1.
[0116] Step 2: Cloning of conserved regions and full-length sequences:
[0117] ① Conserved region amplification primers:
[0118] GDH-F1 5'- GGATC CACGCCGCTCAAGGTC-3' BamHI
[0119] GDH-R1 5'- AAGCTT TACCAAGAAAATCACCGTGGTC-3' HindⅢ
[0120] Primers for full-length sequence amplification:
[0121] GDH-F2 5'-CG GGATCC ATCAAAATGGTCCAGCCTTCC-3' BamHI
[0122] GDH-R2 5'-CCC AAGCTT TTACCAGAAATCACCGTGGTCG-3' HindⅢ
[0123] ② PCR amplification system for the conserved region and full-length sequence of glutamate dehydrogenase gene
[0124]
[0125] ③PCR reaction conditions
[0126] Reaction conditions in the conserved region: pre-denaturation at 95 °C for 5 min; denaturation temperature at 94 °C for 30 s; annealing temperature at 55.6 °C and 58.0 °C for 30 s; extension temperature at 72 °C for 1:30 min; after 35 cycles, at 72 °C Extend for 10 min.
[0127] Reaction conditions for the full-length sequen...
Embodiment 3
[0131] Step 1: Cell preparation:
[0132] With embodiment 1.
[0133] Step 2: Expression of the conserved region and full-length sequence of the enzyme gene:
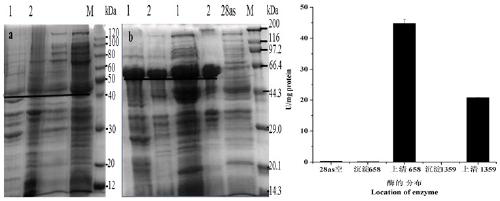
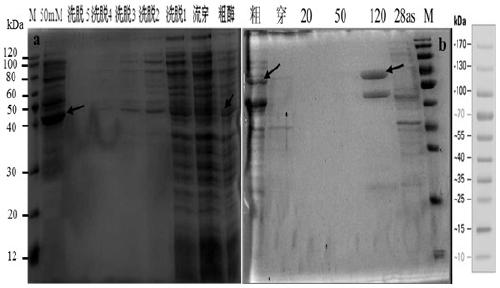
[0134] (1) After the PCR amplification product is recovered, it is ligated with pMD19-T (pMD19-T-658-GDH (conserved region) and pMD19-T-1359-GDH (full-length sequence)) and transformed E. coli DH5α competent cells, pick positive colonies, extract plasmids for PCR detection, and the positive clones have been sequenced. Then the recombinant T vector (pMD19-T-658-GDH (conserved region) and pMD19-T-1359-GDH (full-length sequence)) and the expression vector (pET-28as) were digested with BamHI and HindIII.
[0135]
[0136] (2) React overnight at 37 °C, perform 1% agarose gel electrophoresis, cut the gel to recover the target band, and purify it with the Omega Gel Recovery Kit. Then connect the expression vector, and connect the target fragment and the expression vector after digestion.
[0137]
[0138] Ligation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com