Aeromonas salmonicida inactivated vaccine and applications thereof
A technology for killing Aeromonas salmonids and inactivating vaccines is applied in the directions of antibacterial drugs, medical preparations containing active ingredients, and antibody medical ingredients to achieve the effects of good safety, low cost and simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
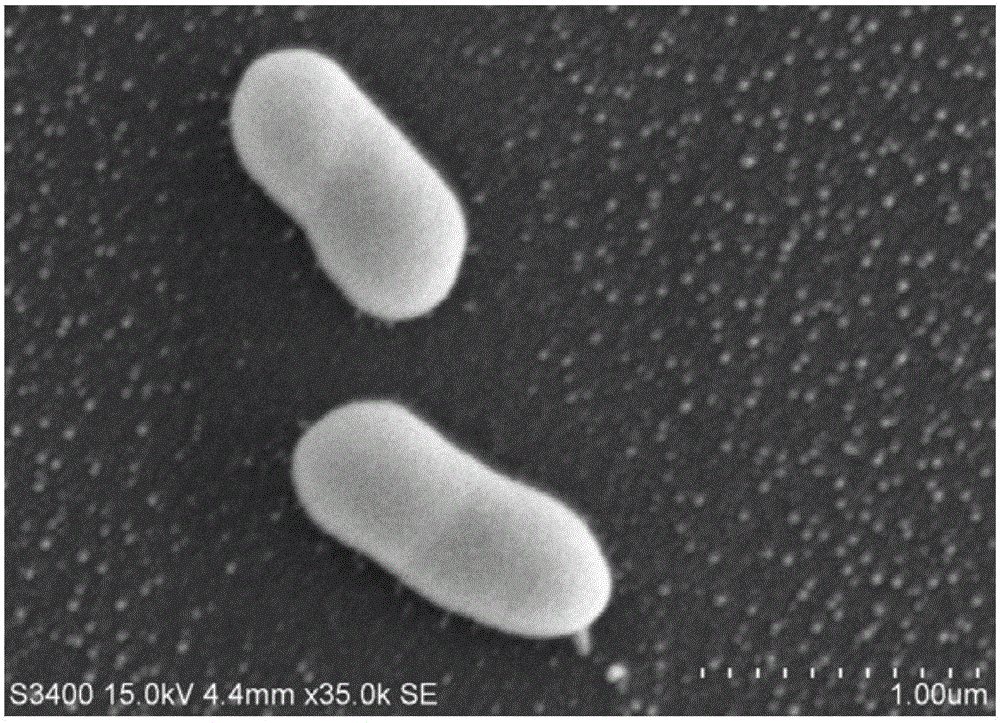
[0035] After the rejuvenation of Aeromonas salmonicida subspecies CGMCC No.7335, pick a single colony and inoculate it into LB liquid medium for 24-48 hours on a shaking table, add a final concentration of 0.3% formaldehyde to inactivate at room temperature for 48 hours, Centrifuge at 5000 rpm for 10 minutes, wash with sterile water, and resuspend the bacteria with sterile water to a concentration of 10 9 cells / ml, the inactivated vaccine antigen is obtained.
[0036] Take 19.2ml of the above-mentioned inactivated vaccine antigen suspension, add 0.8ml of sterilized Tween-80 and mix well to prepare the aqueous phase of the emulsified vaccine; then use 18.8ml of vaccine white oil, 1.2ml of Span-80, 0.4g of stearic acid Mix the aluminum phases, heat to completely dissolve the aluminum stearate, and sterilize to prepare the oil phase of the emulsified vaccine; use a magnetic stirrer at a speed of 5000 rpm, slowly add an equal volume of the oil phase to the water phase, and stir fo...
Embodiment 2
[0038] After the rejuvenation of Aeromonas salmonicida subspecies CGMCC No.7335, pick a single colony and inoculate it into LB liquid medium for 24-48 hours on a shaking table, add a final concentration of 0.3% formaldehyde to inactivate at room temperature for 48 hours, Centrifuge at 5000 rpm for 10 minutes, wash with sterile water, and resuspend the bacteria with sterile water to a concentration of 10 9 cells / ml, the inactivated vaccine antigen is obtained.
[0039]Take 19.6ml of the above-mentioned inactivated vaccine antigen suspension, add 0.4ml of sterilized Tween-80 and mix well to prepare the aqueous phase of the emulsified vaccine; then use 38.4ml of vaccine white oil, 1.6ml of Span-80, 0.6g of stearic acid Mix the aluminum phase, heat to completely dissolve the aluminum stearate, and sterilize to prepare the oil phase of the emulsified vaccine; use a disperser at a speed of 10,000 rpm, slowly add 40ml of the oil phase to 20ml of the water phase, and stir for 3 minute...
Embodiment 3
[0041] Step 1) Vaccination
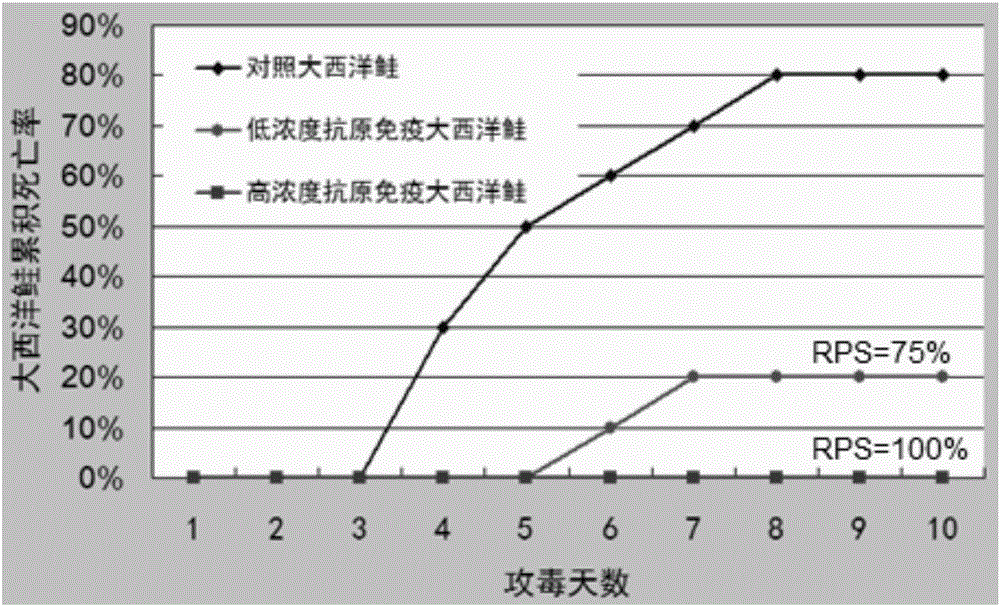
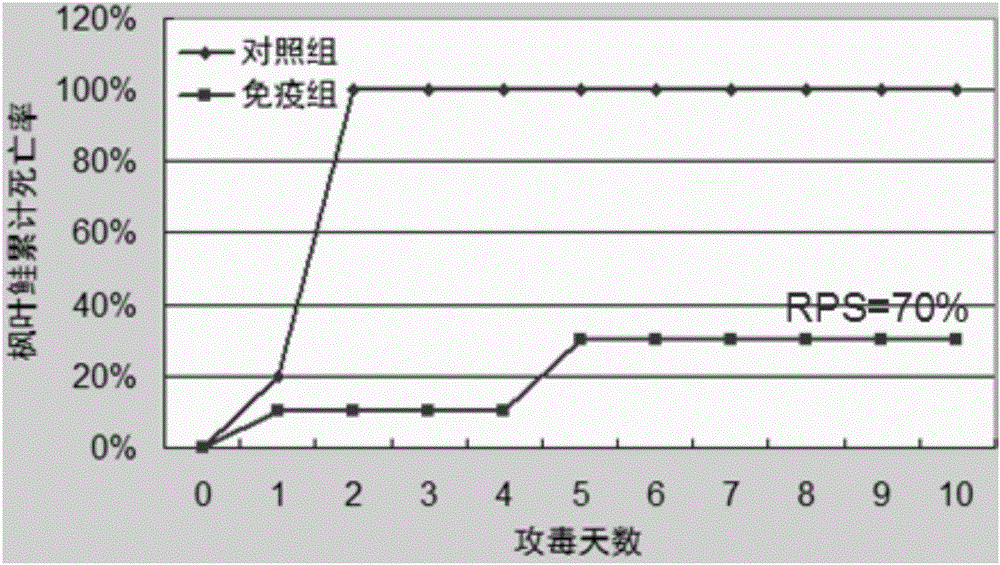
[0042] According to the preparation method of the vaccine in the above-mentioned embodiment 1, the vaccine is prepared, and the inactivated vaccine antigen in the vaccine water phase is respectively provided with a high concentration of vaccine antigen (antigen cell concentration 1 × 10 8 cells / ml), low concentration vaccine antigen (antigen cell concentration 1×10 5 cells / ml) and an antigen-free vaccine without inactivated vaccine antigen (that is, the aqueous phase is sterile water and Tween-80), and an antigen-free vaccine was used as a control.
[0043] 300 Atlantic salmon (body weight 35g-40g) were randomly divided into 3 groups, 100 in each group. These three groups were named high concentration group, low concentration group and control group, respectively. Each Atlantic salmon in the high concentration group was intraperitoneally injected with 100 μl of antigen cells at a concentration of 1×10 8 cells / ml vaccine, intraperitoneally inject...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com