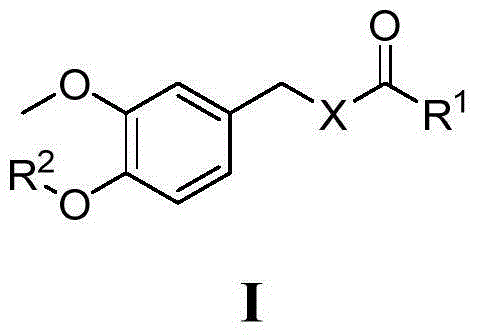
Vanillylamine/vanillyl alcohol derivatives as well as preparation method and application thereof
A technology of vanillol and derivatives, applied in the field of medicine, can solve problems such as the rise of alanine aminotransferase, and achieve the effects of easy synthesis, improved properties and activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] compound i a Synthesis:
[0056]
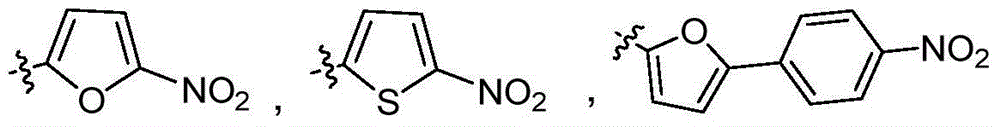
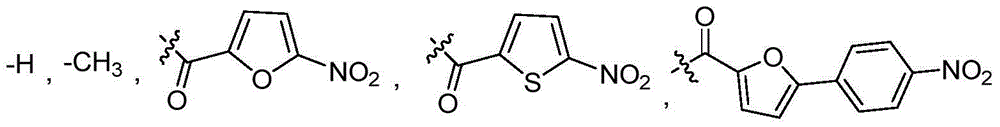
[0057] 5-Nitrofuran-2-carboxylic acid (0.16 g, 1 mmol), vanillylamine hydrochloride (0.19 g, 1 mmol), EDC (0.27 g, 1.5 mmol) and HOBt (0.19 g, 1.5 mmol) were dissolved in N, In N-dimethylformamide (10mL), after stirring for 10min, 3-picoline (0.26mL) was added to the above reaction solution, and after stirring for 18h, 30ml of saturated sodium bicarbonate solution and 30ml of ethyl acetate were added to extract The organic phase was washed three times with saturated brine, dried with anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography (ethyl acetate:petroleum ether=1:2) to obtain the product vanilla amide derivative i a (R 1 =5-nitrofuryl, R 2 =H) 0.20g, yield: 65%; white solid; 1 H NMR (400MHz, DMSO) δ9.29(t, J=5.8Hz, 1H), 8.87(s, 1H), 7.74(d, J=3.9Hz, 1H), 7.43(d, J=3.9Hz, 1H ),6.91(s,1H),6.72(s,2H),4.35(d,J=6.0Hz,2H),3.75(s,3H). 13C NMR (101MHz, DMSO) δ155.93, 151.43, 148.31, 147.41, 145...
Embodiment 2
[0060] compound i b Synthesis:
[0061]
[0062] The synthetic method refers to Example 1, wherein 5-nitrothiophene-2-carboxylic acid is substituted for 5-nitrofuran-2-carboxylic acid to obtain the product vanillic amide derivative i b (R 1 =5-nitrothienyl, R 2 =H)0.48g, yield: 68%; white solid; 1 H NMR (400MHz, DMSO) δ9.59(t, J=5.5Hz, 1H), 8.92(s, 1H), 8.13(d, J=4.3Hz, 1H), 7.94(d, J=4.4Hz, 1H ),6.93(s,1H),6.75(d,J=8.1Hz,2H),4.36(d,J=5.8Hz,2H),3.75(s,3H). 13 CNMR (101MHz, DMSO) δ159.28, 152.80, 147.43, 146.58, 145.71, 130.15, 129.30, 127.44, 120.07, 115.25, 112.13, 55.59, 42.70. MS (ESI) calcd for C 13 h 12 N 2 o 5 S::309.4[M+H] + .
Embodiment 3
[0064] compound i c Synthesis
[0065]
[0066] The synthetic method refers to Example 1, wherein 5-(4-nitrophenyl)-2-furancarboxylic acid is substituted for 5-nitrofuran-2-carboxylic acid to obtain the product vanilla amide derivative i c (R 1 =5-(4-nitrophenyl)-2-furyl, R 2 =H)0.33g, yield: 48%; brown solid; 1 H NMR (400MHz, DMSO) δ9.12(t, 1H), 8.85(s, 1H), 8.32(d, J=8.7Hz, 2H), 8.18(d, J=8.7Hz, 2H), 7.42(d ,J=3.4Hz,1H),7.28(d,J=3.4Hz,1H),6.93(s,1H),6.74(s,2H),4.39(d,J=5.8Hz,2H),3.76(s ,3H). 13 C NMR (101MHz, DMSO) δ157.22, 152.19, 148.70, 147.41, 146.64, 145.56, 135.17, 130.05, 125.07, 124.29, 119.94, 115.88, 115.26, 112.00, 111.53, 9630, 45 19 h 16 N 2 o 6 : 369.5[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com