Preparation method and application of copper-aluminum hydrotalcite material
A hydrotalcite, copper-aluminum technology, applied in chemical instruments and methods, copper compounds, inorganic chemistry, etc., can solve the problems of poor crystal phase and serious agglomeration of hydrotalcite, and achieve excellent electrochemical properties and high atomic conversion rate. , the effect of excellent electrochemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
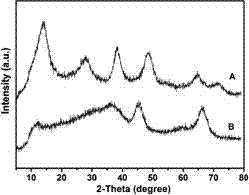
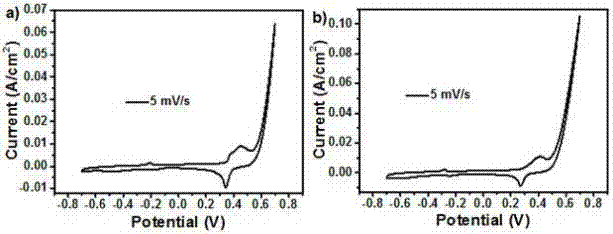
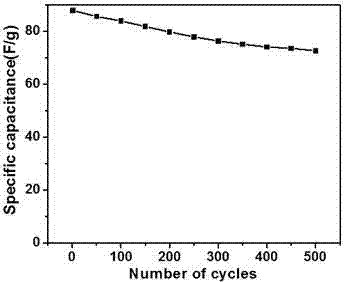
[0024] In this example, see Figure 1~3 , a method for preparing a copper-aluminum hydrotalcite material, comprising the steps of:
[0025] 1) Weigh 0.1 g of sodium carbonate, add it to 10 mL of ammonia solution, stir well, dissolve it in deionized water to 100 mL, and remove the oxygen in the mixed solution with nitrogen gas;
[0026] 2) Weigh 0.96 g of copper nitrate and 7.5 g of aluminum nitrate to prepare a mixed solution of copper nitrate and aluminum nitrate, dissolve it in deionized water to 100 mL, and remove the oxygen in the mixed solution with nitrogen gas;
[0027] 3) Mix 150 mg of sodium citrate with 300 mg of copper nitrate, add 10 ml of ethanol, and add 30 ml of deionized water to prepare a mixed ethanol aqueous solution of sodium citrate and copper nitrate, dissolve sodium citrate and copper nitrate Afterwards, Na is obtained by the reaction 2 [Cu(C 6 h 4 o 7 )] solution, the Na 2 [Cu(C 6 h 4 o 7 )] solution into the three-necked flask, under the prote...
Embodiment 2
[0040] This embodiment is basically the same as Embodiment 1, especially in that:
[0041] In this embodiment, a method for preparing a copper-aluminum hydrotalcite material comprises the following steps:
[0042] 1) Weigh 0.25 g of sodium carbonate, add it to 20 mL of ammonia solution, stir well, dissolve it in deionized water to 100 mL, and remove oxygen in the mixed solution with nitrogen gas;
[0043] 2) Mix 1.8 g of copper nitrate and 14.76 g of aluminum nitrate, dissolve in deionized water to 100 mL, and remove oxygen in the mixed solution with nitrogen gas;
[0044] 3) Mix 200 mg of sodium citrate with 300 mg of copper nitrate, add 15 mL of ethanol, and add 30 ml of deionized water. After dissolving sodium citrate and copper nitrate, Na 2 [Cu(C 6 h 4 o 7 )] solution, the Na 2 [Cu(C 6 h 4 o 7 )] solution into the three-necked flask, under the protection of nitrogen, the mixed solutions prepared in the step 1) and step 2) were passed through the partial pressure f...
Embodiment 3
[0049] This embodiment is basically the same as the previous embodiment, and the special features are:
[0050] In this embodiment, a method for preparing a copper-aluminum hydrotalcite material comprises the following steps:
[0051] 1) Weigh 0.15 g of sodium carbonate, add it to 15 mL of ammonia solution, stir well, dissolve it in deionized water to 100 mL, and remove oxygen in the mixed solution with nitrogen gas;
[0052] 2) Mix 1.5 g of copper nitrate and 12.3 g of aluminum nitrate, dissolve in deionized water to 100 mL, and remove oxygen in the mixed solution with nitrogen gas;
[0053] 3) Mix 200 mg of sodium citrate with 300 mg of copper nitrate, add 15 mL of ethanol, and add 30 ml of deionized water. After dissolving sodium citrate and copper nitrate, Na 2 [Cu(C 6 h 4 o 7 )] solution, the Na 2 [Cu(C 6 h 4 o 7 )] solution into the three-necked flask, under the protection of nitrogen, the mixed solutions prepared in the step 1) and step 2) were passed through th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com