Fusion protein of horseradish peroxidase and antibody fragment and application
A technology of horseradish peroxidase and fusion protein, which is applied in the field of fusion protein and its immunology application, and can solve the problems of decreased biological activity of enzymes and antibodies and low labeling efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: preparation embodiment
[0036] 1. According to the amino acid sequence of the lysozyme nanobody of SEQ ID NO: 1 and the amino acid sequence of SEQ ID NO: 3 horseradish peroxidase, optimize the cDNA of lysozyme and horseradish peroxidase expressed in mammalian cells Sequences SEQ ID NO:2 and SEQ ID NO:4.
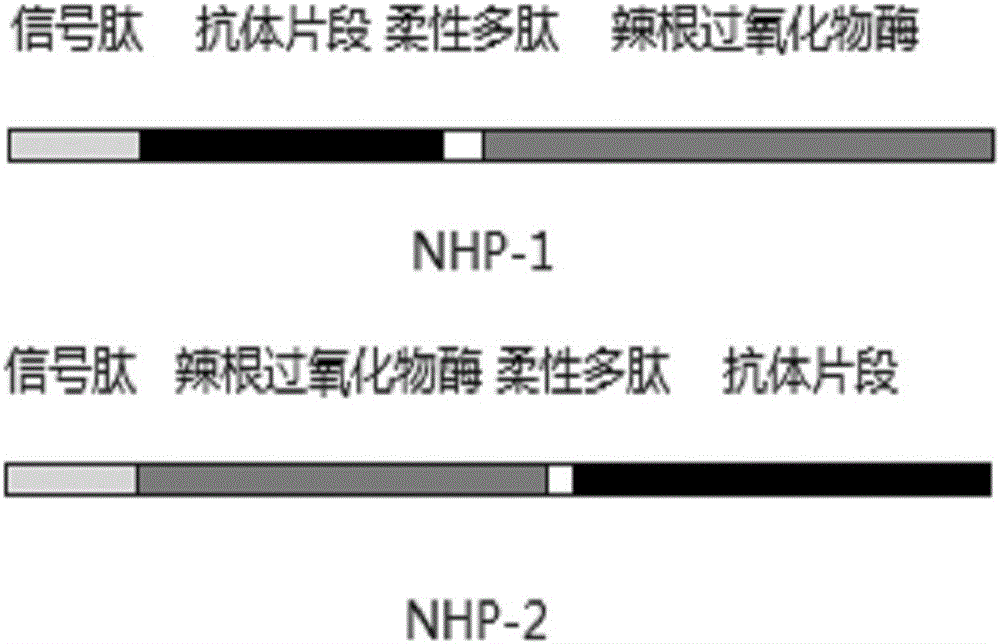
[0037] 2. Adding a flexible linker peptide (GGGGS) to the lysozyme nanobody and horseradish peroxidase sequences 3 , to obtain the DNA sequences SEQ ID NO: 5 and SEQ ID NO: 6 expressing NHP-1 and NHP-2, respectively. Two DNA sequences are artificially synthesized. figure 1 Three connection modes of the fusion protein are shown, and there may also be a signal peptide in the amino acid of the fusion protein, so as to facilitate the secretory expression of the fusion protein.
[0038] 3. Cloning NHP-1 and NHP-2 genes into the EcoRI and HindIII restriction sites of the eukaryotic expression vector pCDNA3.1 respectively to obtain expression vectors pCDNA-N...
Embodiment 2
[0046] Example 2. Detection of fusion protein immune activity
[0047] Take chicken egg white lysozyme, prepare a 10ug / ml solution with PBS, take a 96-well microtiter plate, and coat 100 microliters per well overnight. The next day, wash the wells with PBST, block with 0.5% BSA solution for 1 hour, take 100 microliters of transfected 293T cell culture medium supernatant, add to the wells, place in the dark at room temperature for 1 hour, wash with PBST 3 times, Add 100 microliters of DAB chromogenic substrate, react in the dark for 15 minutes at room temperature, and visually observe the color development (see Figure 4 ). Figure 4Middle, 1. Blank control NHP-1 transfected 293T cell supernatant; 2. Lysozyme-coated 4× diluted NHP-1 transfected 293T cell supernatant; 3. Lysozyme-coated 10× diluted NHP-1 transfected 293T Cell supernatant; 4. Lysozyme-coated 4× diluted NHP-2 transfected 293T cell supernatant; 5. Lysozyme-coated 10× diluted NHP-2 transfected 293 cell supernatant...
Embodiment 3
[0049] Example 3. Horseradish peroxidase chemical labeling of Nanobodies
[0050] Dissolve 5mg horseradish peroxidase in 0.5ml 0.1mol / L NaHCO 3 solution; add 0.5ml 10mmol / L NaIO 4 solution, mix well, tightly cap the cork, and protect from light at room temperature for 2 hours. Add 0.75ml 0.1mol / L Na 2 CO 3 Mix well, then add 0.75ml of purified anti-lysozyme nanobody (15mg / ml), and mix well. Weigh 0.3g of Sephadex G25 dry powder and add it into the outer cylinder of a 5ml syringe with a glass wool pad on the lower mouth; then transfer the above-mentioned cross-linked product into the outer casing of the syringe; cover tightly and act at room temperature (protected from light) for 3 hours or overnight at 4°C. Use a little PBS to wash out all the cross-linked products, collect the eluate, add 1 / 20 volume of freshly prepared 5mg / ml NaBH4 solution, mix well, and act for 30 minutes at room temperature; then add 3 / 20NaBH 4 solution, mix well, and act at room temperature for 1 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com