Method for producing and method for processing complex fluoride phosphor
一种复合氟化物、制造方法的技术,应用在化学仪器和方法、发光材料等方向,达到耐湿性优异的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
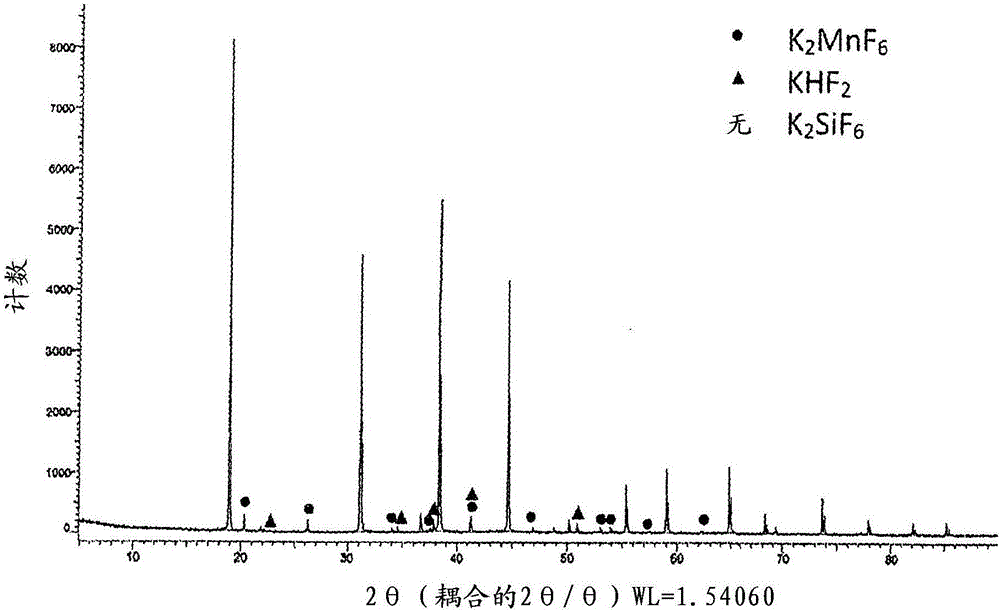
[0086] Potassium silicofluoride (manufactured by Morita Chemical Industry Co., Ltd., K 2 SiF 6 ) powder 26.43g and potassium hexafluoromanganate (made by the method described in Reference Example 1 described later, K 2 MnF 6 ) powder 2.46g was put into the same polyethylene zipper bag. Shake or swirl slowly by hand for 5 minutes to mix. The mixing ratio is such that Mn corresponds to 0.083 mol with respect to 1 mol of Si.
[0087] Potassium hydrogen fluoride (acid potassium fluoride manufactured by STELLA CHEMIFA, KHF 2 ) powder 14.06g, mixed in the same manner as above. Ratio is 1 mole relative to Si, KHF 2 Equivalent to 1.5 moles. 2.0 g (mixed powder) was taken out from the powder mixture for later evaluation.
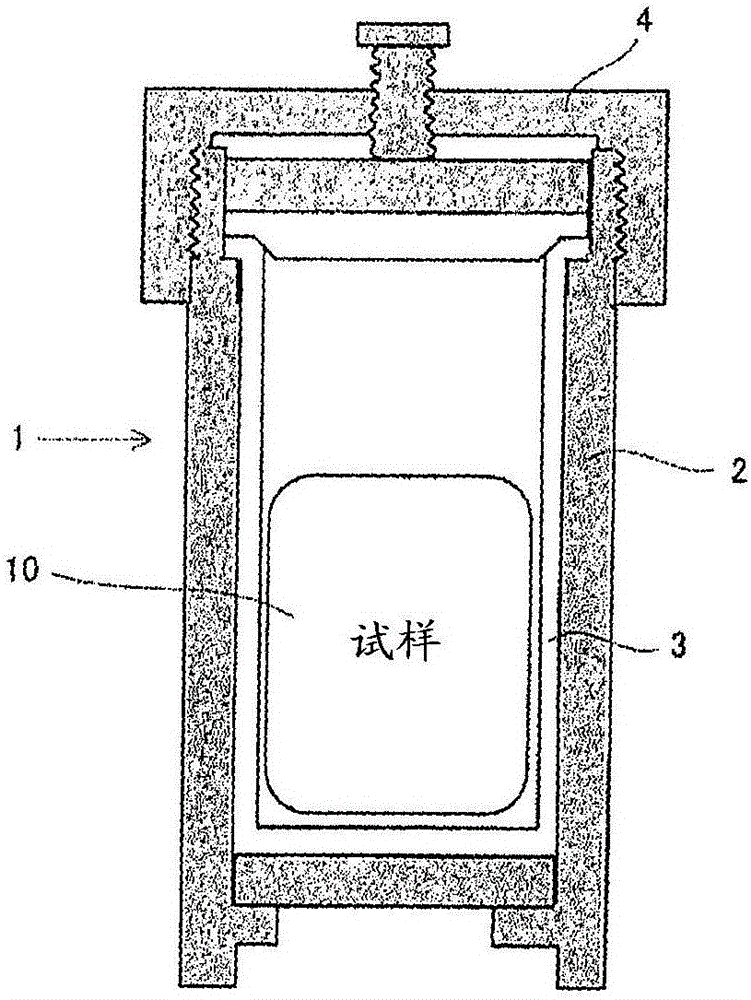
[0088] Put the powder mixture in figure 1 Airtight in the double container 1 shown in . here, figure 1 Among them, the double container 1 is formed by forming the inner layer 3 made of polytetrafluoroethylene on the inner wall of the container body 2 made ...
reference example 1
[0101] [K 2 MnF 6 preparation]
[0102] According to the method described in Non-Patent Document 4, it was prepared by the following method.
[0103] A spacer (diaphragm) of a fluororesin-based ion-exchange membrane was installed in the center of a reaction tank made of vinyl chloride resin, and an anode and a cathode made of platinum plates were installed in each of the two chambers sandwiching the ion-exchange membrane. An aqueous solution of hydrofluoric acid for dissolving manganese (II) fluoride is placed on the anode side of the reaction tank, and an aqueous solution of hydrofluoric acid is placed on the cathode side. Both electrodes were connected to a power supply, and electrolysis was performed at a voltage of 3V and a current of 0.75A. After completion of the electrolysis, a solution of potassium fluoride saturated in an aqueous hydrofluoric acid solution was added in excess to the reaction liquid on the anode side. The yellow solid product that generates is filt...
Embodiment 2
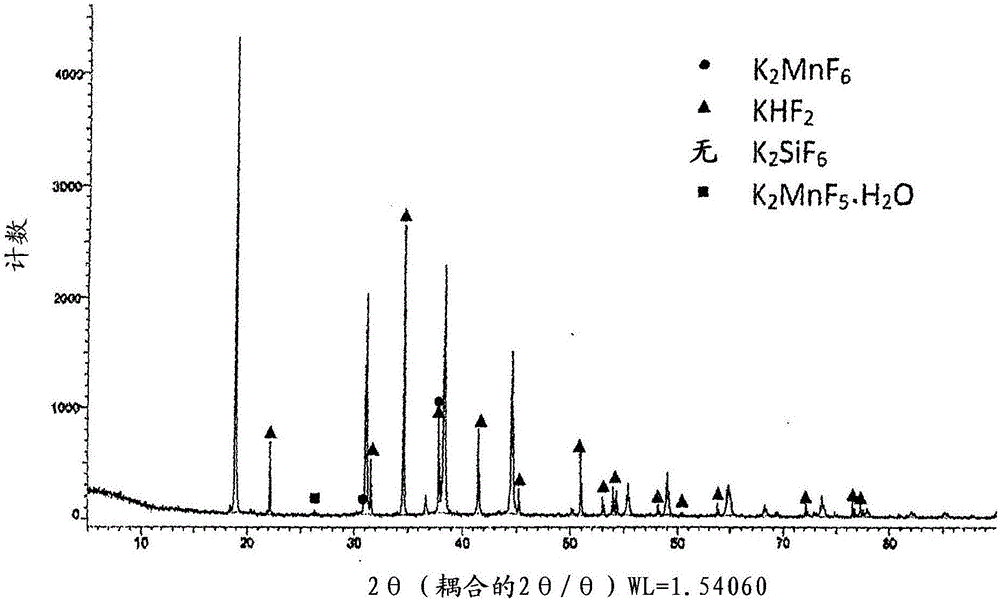
[0105] 14.06g of potassium hydrogen fluoride is replaced by 11.72g, and 4.08g of potassium hydrogen sulfate (Wako Pure Chemicals special grade, KHSO 4 ), except that, operate in the same manner as in Example 1, and obtain K of 31.0g 2 SiF 6 : Powder product of Mn. As a result of the particle size distribution measured in the same manner as in Example 1, D10 = 8.2 μm, D50 = 22.1 μm, and D90 = 35.4 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com