Method for rapidly measuring purity of bulk pharmaceutical chemical of lincomycin hydrochloride on basis of hydrogen-nuclear magnetic resonance
A lincomycin hydrochloride and nuclear magnetic resonance technology, applied in the field of medical analysis, can solve the problems of affecting the measurement results, pollution or degradation, high price, etc., and achieve the effects of short analysis time, simple pretreatment, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
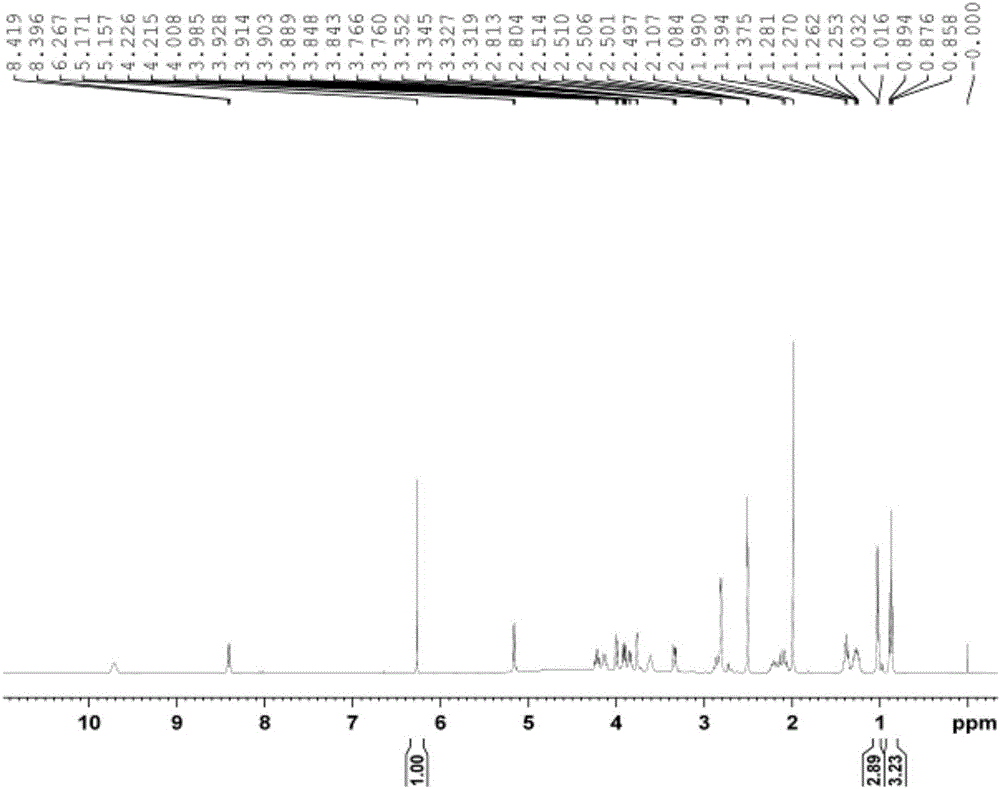
[0046] Accurately weigh 10.733 mg of lincomycin hydrochloride sample and 4.706 mg of maleic acid, and add 600 μL and 250 μL DMSO-d respectively 6 After dissolving, transfer 450 μL lincomycin hydrochloride solution and 50 μL maleic acid solution to NMR tubes for 1 H NMR test. The resonant frequency of NMR spectrometer is 400MHz, 90°pulse, d 1 is 100s, the number of sampling accumulations is 32, the spectral width is 20ppm, the test temperature is 28°C, and the AQ is 4.1s.
[0047] exist 1 In the H NMR spectrum, the internal standard maleic acid δ H 6.27 Signal peak integral value is 1, lincomycin hydrochloride δ H The integral value of the signal at 0.88 is 3.23. According to formula-1, the purity of lincomycin hydrochloride can be calculated to be 99.0%, repeated 5 times, and the relative standard deviation is 0.3%.
[0048] W%=(m IS ×H IS ×A S × M S ×100%) / (M IS ×A IS ×H S × m S ) (Formula 1)
[0049] W%—purity of lincomycin hydrochloride
[0050] m IS - the...
Embodiment 2
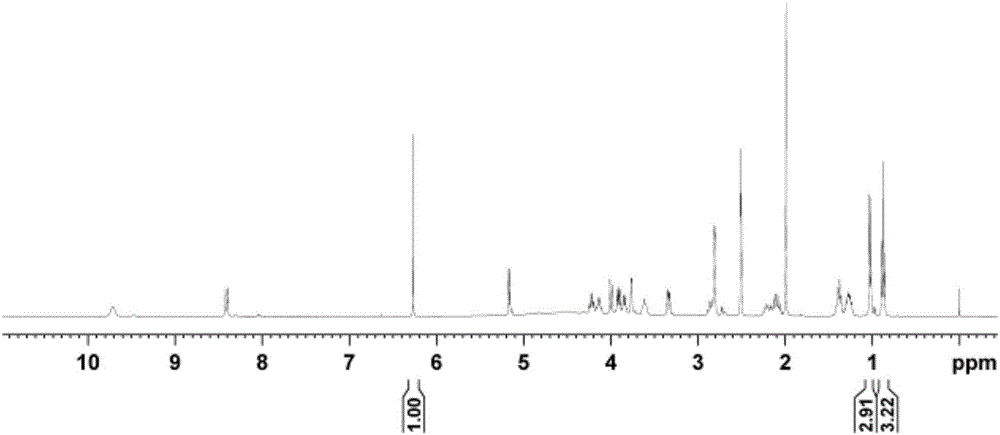
[0059] 1 The H NMR test sample is the same as in Example 1. The resonant frequency of NMR spectrometer is 400MHz, 90°pulse, d 1 is 80s, the number of sampling accumulations is 32, the spectral width is 20ppm, the test temperature is 28°C, and the AQ is 2.0s.
[0060] exist 1 In the H NMR spectrum, the internal standard maleic acid δ H 6.27 Signal peak integral value is 1, lincomycin hydrochloride δ H The integral value of the signal at 0.88 is 3.22. According to formula-1, the purity of lincomycin hydrochloride can be calculated to be 98.7%, repeated 5 times, and the relative standard deviation is 0.2%.
Embodiment 3
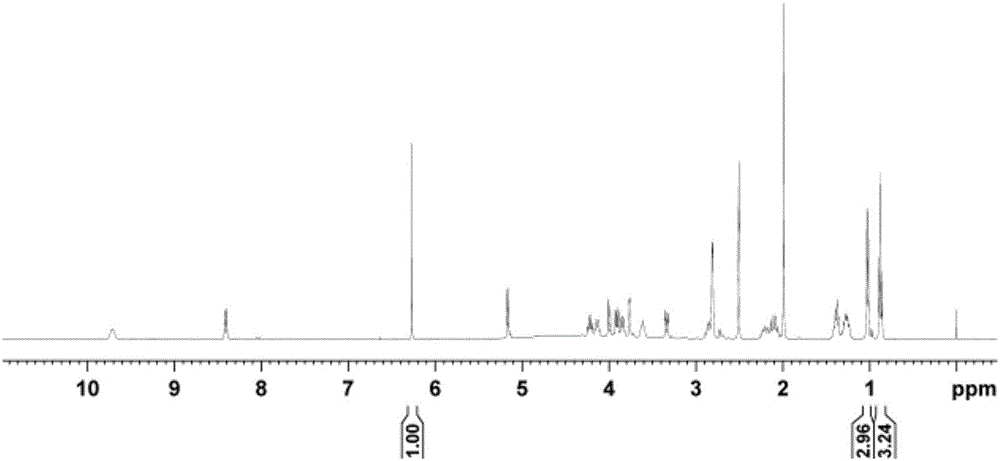
[0062] 1 The H NMR test sample is the same as in Example 1. The resonant frequency of NMR spectrometer is 400MHz, 40°pulse, d 1 is 50s, the number of sampling accumulations is 64, the spectral width is 20ppm, the test temperature is 28°C, and the AQ is 4.1s.
[0063] exist 1 In the H NMR spectrum, the internal standard maleic acid δ H 6.27 Signal peak integral value is 1, lincomycin hydrochloride δ H The integral value of the signal at 0.88 is 3.24. According to formula-1, the purity of lincomycin hydrochloride can be calculated to be 99.3%, repeated 5 times, and the relative standard deviation is 0.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com