Cyclohexanone ammoximation method
A technology of cyclohexanone ammonia oxime and cyclohexanone, which is applied in oxime preparation, organic chemistry, etc., can solve the problems of poor separation effect, serious influence of catalyst diffusion, easy blockage of catalyst and other problems, so as to solve the problem of easy deactivation of catalyst , reduce subsequent separation costs, and achieve good mass transfer between phases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 (comparative example)
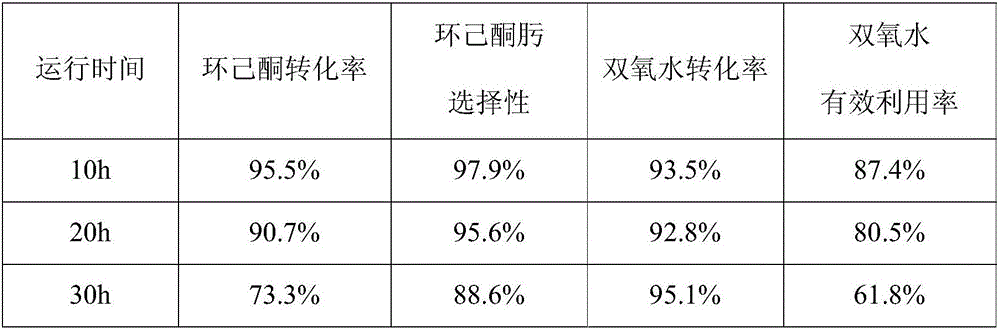
[0042]With reference to the reaction method disclosed in the prior art (patent CN1432560A), the cyclohexanone ammoximation reaction process is designed by itself. Wherein, the raw material ratio is: the molar ratio of hydrogen peroxide to cyclohexanone is 1.1:1, and the molar ratio of ammonia to cyclohexanone is 2.3:1. The reaction conditions are as follows: the temperature is 90° C., the absolute pressure is 0.4 MPa, the catalyst is 3 g (40 mesh) of titanium-silicon molecular sieve formed by tableting, and the contact time between the reaction raw material and the catalyst is 60 minutes. The slurry after the reaction is centrifuged, and the separated catalyst is dried, calcined and weighed. The reaction results are shown in Table 1.
[0043] Table 1
[0044]
[0045] Catalyst mass after reaction: 2.5g.
[0046] This example verifies that in the traditional liquid-phase ammoximation reaction, there is the problem of complicate...
Embodiment 2
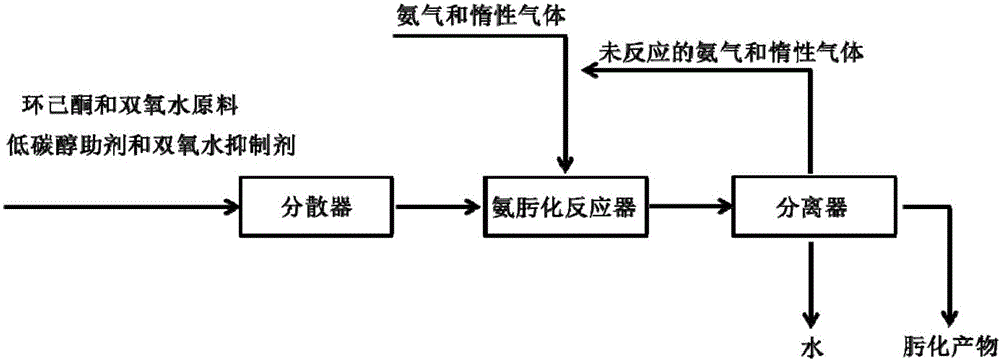
[0048] All reaction parameters and raw material ratios were kept constant in Example 1. In a fixed bed reactor, just follow the attached figure 1 The procedure for cyclohexanone ammoximation test is carried out. The dispersion method of the raw material mixture is high-pressure dispersion, nitrogen is used as the inert gas carrier gas, the boost pressure is 0.1 MPa, and the volume ratio of nitrogen carrier gas to ammonia is 10:1. The reaction results are shown in Table 2.
[0049] Table 2
[0050]
[0051] Catalyst mass after reaction: 3g.
[0052] This example successfully demonstrates that in the solid-phase reaction of ammonia oximation gas, the product and the catalyst can be separated in situ, there is no problem of catalyst loss, the life of the catalyst is greatly extended, and the effective utilization rate of hydrogen peroxide is as high as 90%.
Embodiment 3
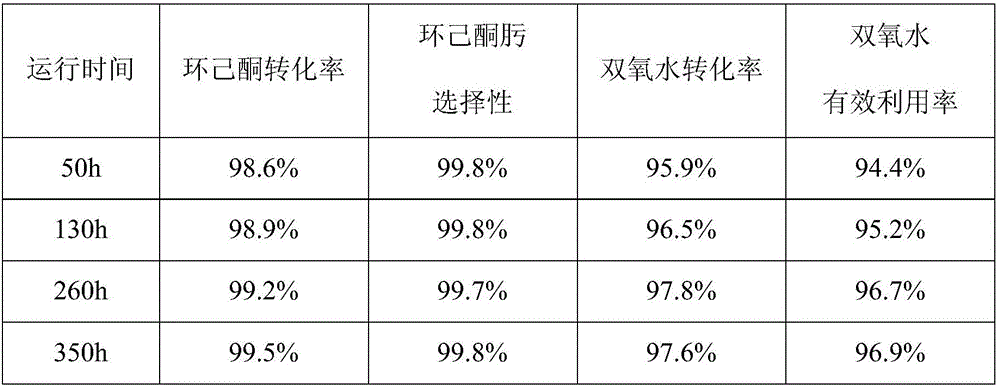
[0054] Repeat Example 2, just replace the oximation reactor with a fluidized bed reactor. The pressure of the booster gas is 0.6MPa, and the catalyst is TS-1 after spray granulation, which is mixed with SiO in the binder 2 The weight ratio of the catalyst is 60:40, the particle size range is 20-120 μm, and the loading amount of the catalyst is 50 g, accounting for about 80% of the total particle weight. The modulation reaction temperature is 120° C., the reaction pressure is normal pressure, helium is used as carrier gas, and the volume ratio of helium to ammonia is 60:1. The reaction results are shown in Table 3.
[0055] table 3
[0056]
[0057] Catalyst mass after reaction: 49.5 g.
[0058] There is basically no loss of the catalyst, the service life of the catalyst is greatly extended, and the effective utilization rate of hydrogen peroxide is as high as 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystal size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com