Sodium lithium titanate nanowire and preparation method thereof
A sodium nano and lithium titanate technology, applied in titanate, nanotechnology, nanotechnology and other directions, can solve problems such as poor electrochemical performance, and achieve the effects of excellent performance, good capacity retention, and high initial discharge specific capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
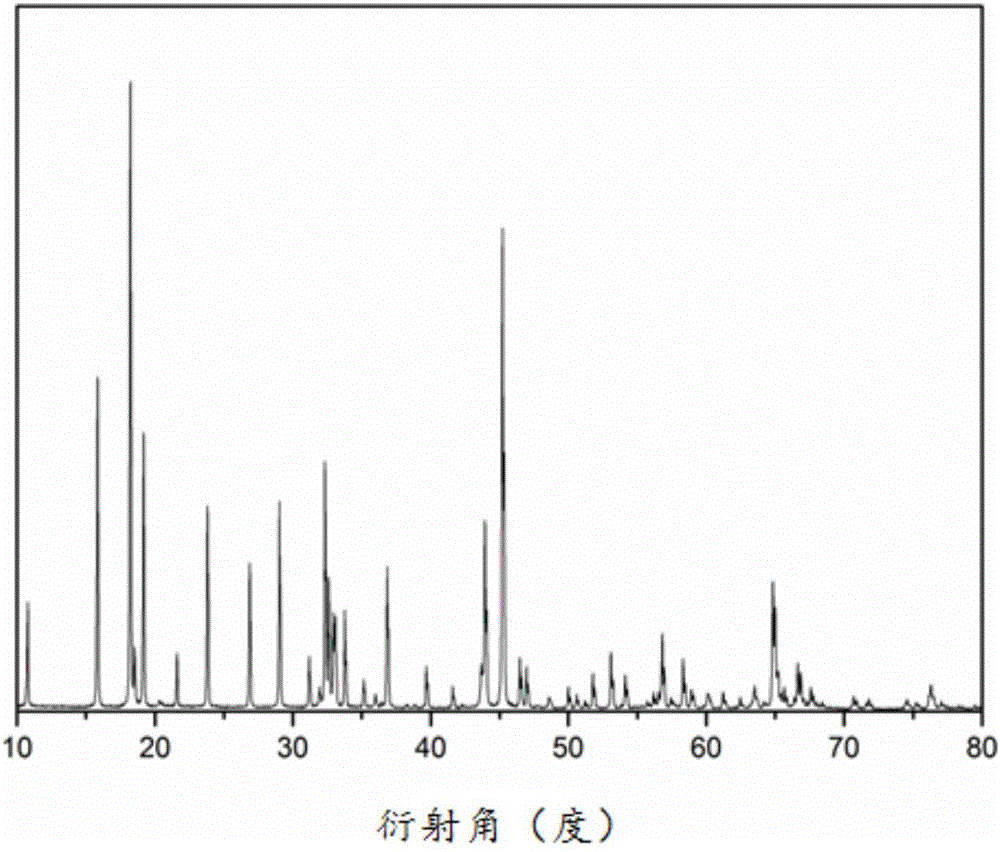
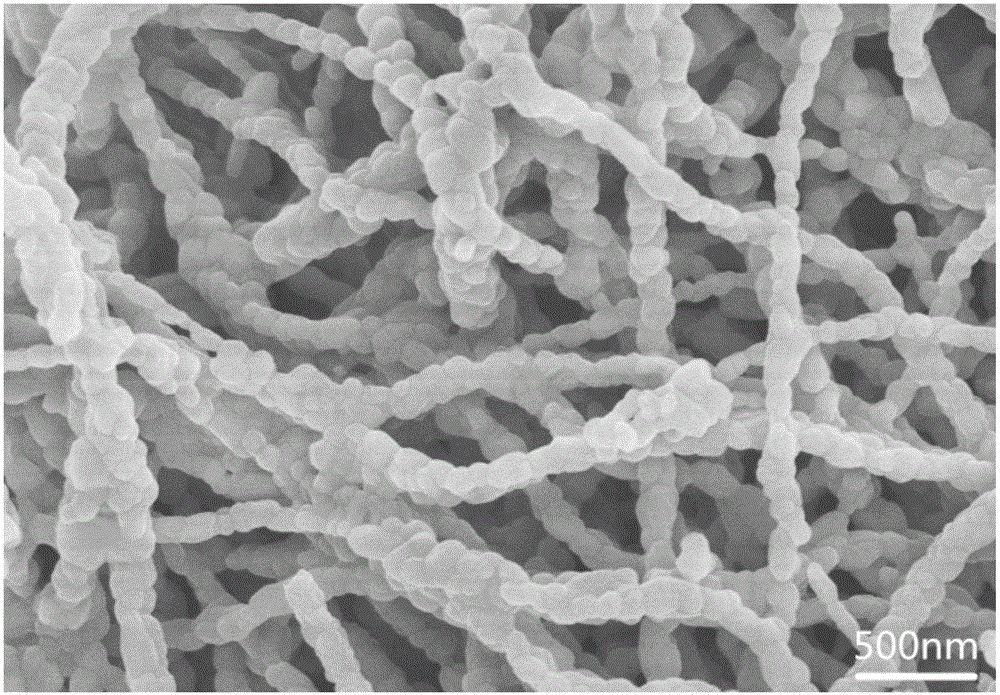
[0021] 0.15mmol of NaCH 3 COO was dissolved in 2 mL dimethylformamide (DMF) to form solution A; 0.15 mmol LiCH 3 Dissolve COO in 5 mL of absolute ethanol to form solution B; dissolve 0.3 mmol of isopropyl titanate in 3 mL of dimethylformamide (DMF) to form solution C; mix solutions A, B and C evenly and add 2 mL of glacial acetic acid and 1.2g polyvinyl alcohol, stirred for 2h to form a clear solution D; the clear solution D was electrospun at a voltage of 15kv and a flow rate of 0.8mL / h; the obtained electrospun product was dried in an oven at 60°C 4h; transfer the dried electrospinning product to a muffle furnace and sinter at 600°C for 6h to obtain lithium sodium titanate NaLiTiO 3 Nanowires. The nanowires were characterized by powder diffraction (XRD), as figure 1 shown. Observe the morphology of the nanowires with a scanning electron microscope, such as figure 2 shown.
Embodiment 2
[0023] 0.15mmol of NaHCO 3 Dissolve in 2mL dimethylformamide (DMF) to form solution A; dissolve 0.225mmol of LiF in 5mL of absolute ethanol to form solution B; dissolve 0.525mmol of isopropyl titanate in 3mL of dimethylformamide ( DMF) to form solution C; after mixing solutions A, B and C evenly, add 1.5mL glacial acetic acid and 1.1g polyvinyl alcohol, stir for 2h to form clear solution D; put clear solution D at a voltage of 20kv and a flow rate of 1.2 Electrospinning was carried out at mL / h; the obtained electrospinning product was dried in an oven at 90°C for 4 hours; the dried electrospinning product was transferred to a muffle furnace, and sintered at 800°C for 3 hours to obtain a lithium-ion battery negative electrode Material lithium sodium titanate NaLiTiO 3 Nanowires.
Embodiment 3
[0025] 0.15mmol of NaCH 3 COO was dissolved in 2 mL of dimethylformamide (DMF) to form solution A; 0.15 mmol of LiNO 3 Dissolve in 5mL of absolute ethanol to form solution B; dissolve 0.45mmol of isopropyl titanate in dimethylformamide (DMF) to form solution C; mix solutions A, B, and C evenly and add 1.5mL of glacial acetic acid and 1.2g polyvinyl alcohol, stirred for 2h to form a clear solution D; the clear solution was electrospun at a voltage of 18kv and a flow rate of 1.0mL / h; the obtained electrospun product was dried in an oven at 80°C for 4h ; Transfer the dried electrospinning product to a muffle furnace and sinter at 700°C for 5 hours to obtain lithium sodium titanate NaLiTiO, a negative electrode material for lithium-ion batteries. 3 Nanowires.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com