Process of producing H2 by way of parallel Fe2+ anodic oxidation and cathodic reduction
A technology of anodic oxidation and process method, which is applied in chemical instruments and methods, photographic process, photographic auxiliary process, etc., and can solve problems such as difficult regeneration, high cell voltage, solvent recovery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
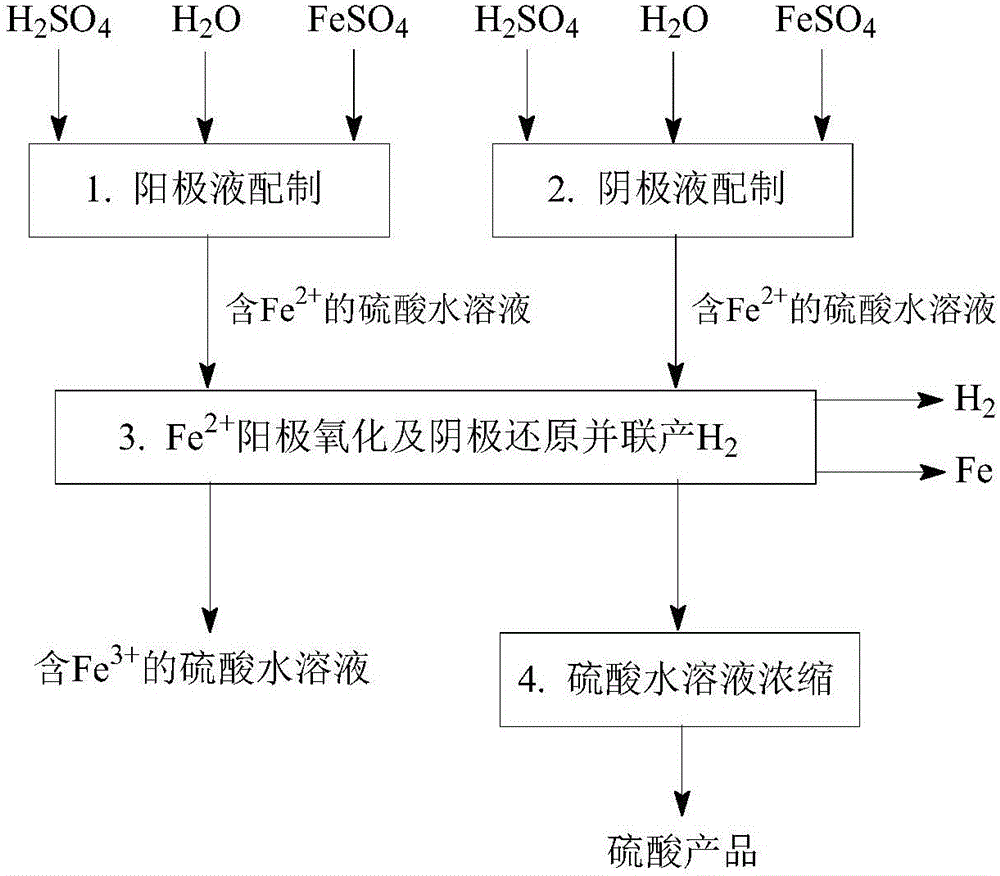
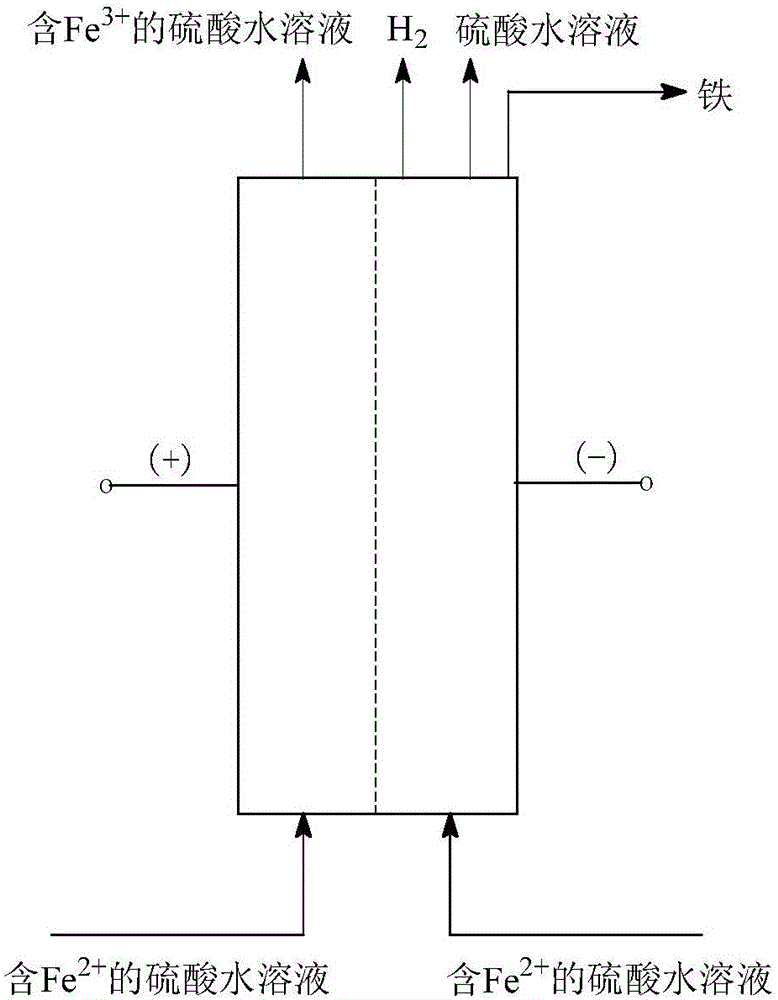
[0092] Such as figure 1 and figure 2 As shown, a Fe 2+ Anodic oxidation and cathodic reduction co-produce H 2 The process method, the concrete steps are as follows:
[0093] (1) Preparation of anolyte: mix sulfuric acid with water to obtain an aqueous solution of sulfuric acid, then dissolve ferrous sulfate in the aqueous solution of sulfuric acid to obtain a solution containing 0.2mol / L H 2 SO 4 and 0.2mol / L Fe 2+ The anolyte, the operating temperature is 20 ℃.
[0094] (2) catholyte preparation: will contain Fe 3+ The sulfuric acid aqueous solution is mixed with sulfuric acid and water, and then the ferrous sulfate is dissolved in the sulfuric acid aqueous solution to obtain 8.0mol / L H 2 SO 4 , 2.0mol / L Fe 2+ and 0.02mol / L Fe 3+ The catholyte, the operating temperature is 20 ℃.
[0095] (3) Fe 2+ Anodic oxidation and cathodic reduction co-produce H 2 : In an electrochemical reactor with a sulfonic acid type cation exchange membrane as the isolation membrane, su...
Embodiment 2
[0097] Such as figure 1 and figure 2 As shown, a Fe 2+ Anodic oxidation and cathodic reduction co-produce H 2 The process method, the concrete steps are as follows:
[0098] (1) Anolyte preparation: will contain Fe 3+The sulfuric acid aqueous solution is mixed with sulfuric acid and water, and then ferrous sulfate is dissolved in the solution to obtain 8.0mol / L H 2 SO 4 , 2.0mol / L Fe 2+ and Fe 3+ For anolyte with concentration ≤0.2mol / L, the operating temperature is 60°C.
[0099] (2) catholyte preparation: will contain Fe 3+ The sulfuric acid aqueous solution is mixed with sulfuric acid and water, and then ferrous sulfate is dissolved in the solution to obtain 8.0mol / L H 2 SO 4 , 2.0mol / L Fe 2+ and 0.02mol / L Fe 3+ The catholyte operating temperature is 60°C;
[0100] (3) Fe 2+ Anodic oxidation and cathodic reduction co-produce H 2 : In an electrochemical reactor with a sulfonic acid type cation exchange membrane as a separator, sulfuric acid as a supporting el...
Embodiment 3
[0102] Such as figure 1 and figure 2 As shown, a Fe 2+ Anodic oxidation and cathodic reduction co-produce H 2 The process method, the concrete steps are as follows:
[0103] (1) Anolyte preparation: will contain Fe 2+ and Fe 3+ The sulfuric acid aqueous solution is directly used as the anolyte, and the solution composition is 6.0mol / L H 2 SO 4 , 0.8mol / L Fe 2+ and 0.1mol / L Fe 3+ , operating temperature is 40°C.
[0104] (2) catholyte preparation: will contain Fe 2+ and Fe 3+ The sulfuric acid aqueous solution is directly used as the catholyte, and the solution composition is 8.0mol / L H 2 SO 4 , 1.0mol / L Fe 2+ and 0.02mol / L Fe 3+ , operating temperature is 40°C.
[0105] (3) Fe 2+ Anodic oxidation and cathodic reduction co-produce H 2 : In a three-dimensional fixed-bed electrode electrochemical reactor with a sulfonic acid-type cation-exchange membrane as a separator, sulfuric acid as a supporting electrolyte, and a graphite electrode as an anode, the operatin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com