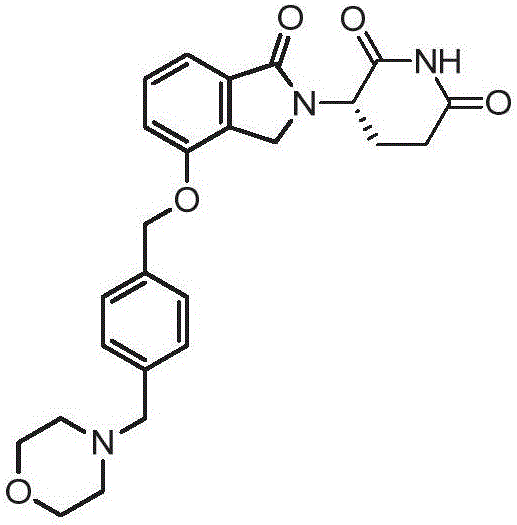
Formulations of (s)-3-(4-((4-(morpholinomethyl)benzyloxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione
A technology of dosage and excipients, applied in (S)-3-(4-((4-(morpholinylmethyl)benzyloxy)-1-oxoisoindoline-2-yl ) Preparation field of piperidine-2,6-dione
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0283] 5.1 Example 1: Compound A Dosage Capsules
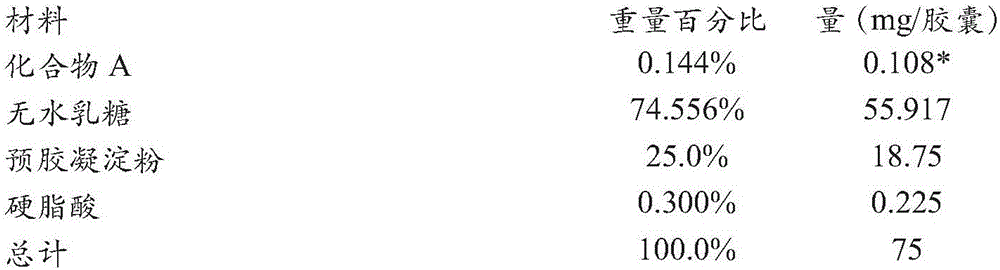
[0284] Table 1 illustrates the batch formulation and single dose formulation of a single dosage unit of Compound A at 0.3 mg strength in #4 capsules.
[0285] Table 1. Formulation of 0.3 mg Strength Compound A Capsules
[0286]
[0287] *Denotes the amount of the salt form of Compound A that provides the potency of 0.3 mg of the free base of Compound A (ie, the amount that provides 0.3 mg of 100% pure Compound A).
[0288] Compound A was premixed with a portion of anhydrous lactose and pregelatinized starch. Pass the premix through a 0.032 inch / 20 mesh screen. Grind the remaining lactose through a 0.032 inch / 20 mesh screen. Combine premix with remaining lactose. To this mixture was further blended stearic acid which passed through a 0.0232 inch / 30 mesh screen. The final blend was enclosed in #4 capsules.
[0289] Table 2 illustrates batch and single dose formulations of single dosage units of 0.1 mg strength Compound...
Embodiment 2
[0304] 5.2 Example 2: Stability of formulations
[0305] The accelerated stability of the 0.3 mg strength PD01-082 formulation (described in Table 1 above) and the other 0.3 mg strength formulations described in Tables 5-7 below were evaluated at 40°C / 75% RH and determined initial , 1-month, 3-month and 6-month time periods impurity levels. To determine impurity levels, the HPLC gradient method was employed using the following conditions:
[0306] Column: XBridge C18 column, 4.6×150mm, 3.5μm particle size
[0307] Temperature: autosampler: ambient temperature; column: 40°C
[0308] Mobile phase: A: 20mM ammonium acetate: acetonitrile (95:5, v / v)
[0309] B: 20mM ammonium acetate: acetonitrile (10:90, v / v)
[0310]
[0311] Flow rate: 1.0mL / min
[0312] Injection volume: 50μL
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com