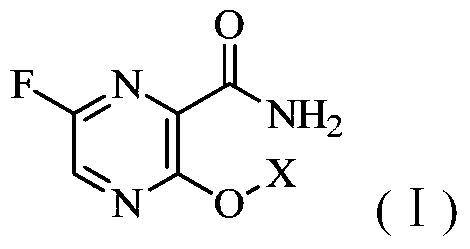
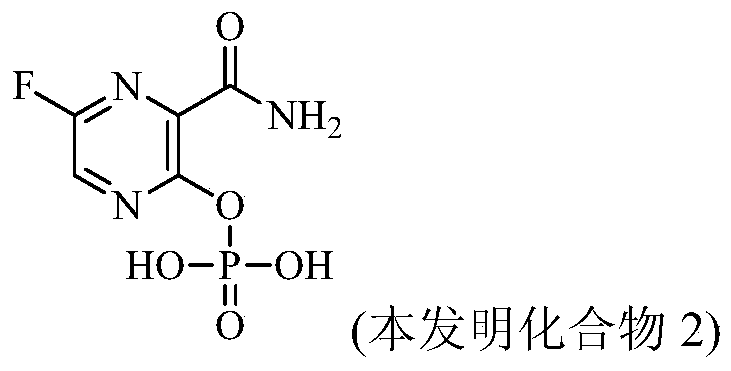
Pyrimidine amide derivatives and their salts
A technology for drugs and compounds, applied in the field of medicine, can solve the problems of cumbersome process, long time consumption, crystallization of preparations, etc., and achieve the effects of improving low solubility, reducing potential safety hazards and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
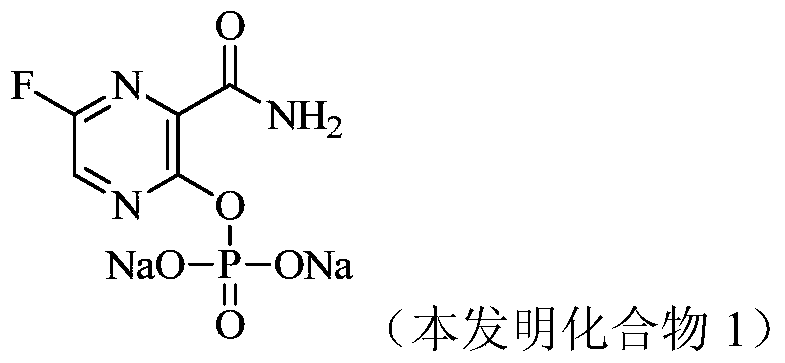
[0073] Example 1 Preparation of 3-carbamoyl-5-fluoropyrazin-2-yl phosphate disodium (compound 1)
[0074] 1. Preparation of dibenzyl (3-carbamoyl-5-fluoropyrazin-2-yl) phosphate
[0075]
[0076] Add N,N-dimethylformamide (5mL) and 6-fluoro-3-hydroxypyrazine-2-carboxamide (200mg, 1.27mmol) into a 10mL two-necked flask, NaH (60%, 203mg, 5.08mmol) was added in batches, then stirred for 1 hour, and tetrabenzyl diphosphate (820mg, 1.52mmol) was added in batches. After the addition was complete, stirring was continued at room temperature for 3 hours. The solid was filtered and the crude product was purified by medium pressure preparation (mobile phase: CH 3 CN,H 2 O,1%NH 4 HCO 3 ), the fractions were collected and freeze-dried to obtain 192 mg of the product (36% yield) as a yellow solid.
[0077] 2. Preparation of disodium 3-carbamoyl-5-fluoropyrazin-2-ylphosphate
[0078]
[0079] Add dibenzyl (3-carbamoyl-5-fluoropyrazin-2-yl) phosphate (192 mg, 0.46 mmol) and dichl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com