A kind of synthetic method of methylcyclopropene probe
A technique for the synthesis of methylcyclopropene, which is applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problems of high price, and achieve the effects of low cost, good application prospects, and easy availability of reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
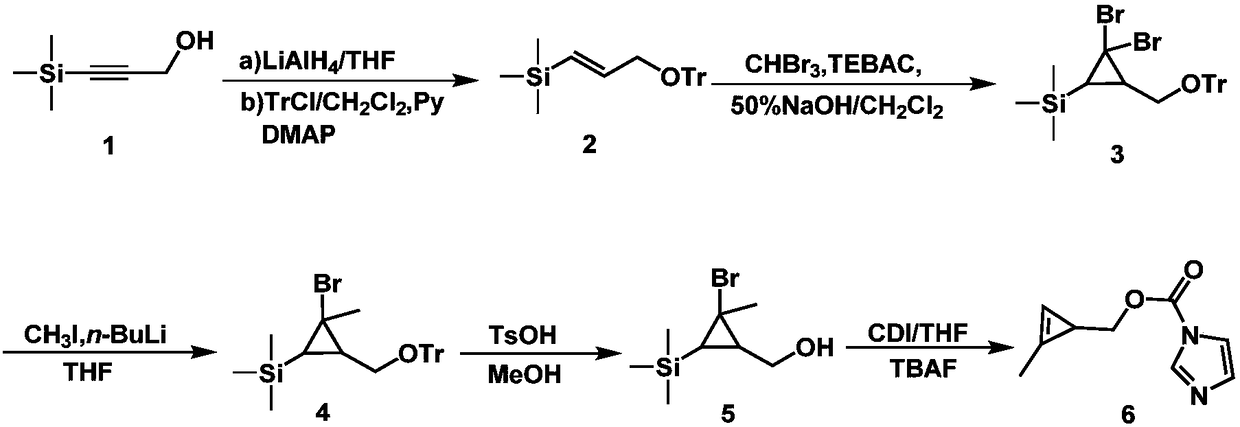
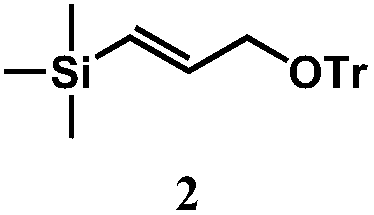
[0020] 1. Add 9.8g (76mmol) trimethylsilylpropynyl alcohol and 10.9mL (38mmol) lithium aluminum hydride into 200mL tetrahydrofuran, stir at 0°C for 16 hours, add 5mL saturated ammonium chloride aqueous solution to quench the reaction, and then extract with ether for 3 Once, the organic phase was washed with a saturated NaCl aqueous solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain trimethylsilylpropenol; 12g (92mmol) trimethylsilylpropenol, 30g (110mmol) triphenylchloromethane, 1.1 g (9.2mmol) of 4-dimethylaminopyridine and 14.8mL (18.4mmol) of pyridine were added to 200mL of dichloromethane, stirred at room temperature for 12 hours, and then 50mL of 1mol / L hydrochloric acid was added, followed by washing with saturated NaCl aqueous solution, dichloromethane After extraction, drying over anhydrous sodium sulfate, and concentration under reduced pressure, compound 2 was obtained with a yield of 75%.
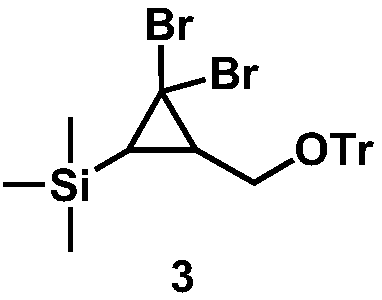
[0021] 2. Dissolve 4.3g (11.5m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com