Synthesis method of 2-aminoindane derivative and product of 2-aminoindane derivative
A synthesis method and aminoindan technology are applied in the preparation of aminohydroxy compounds, the preparation of amino compounds from amines, the preparation of carbon-based compounds, etc., and can solve the problems of difficult operation, harsh reaction conditions, and complicated reaction steps, and achieve easy operation, The effect of mild reaction conditions and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
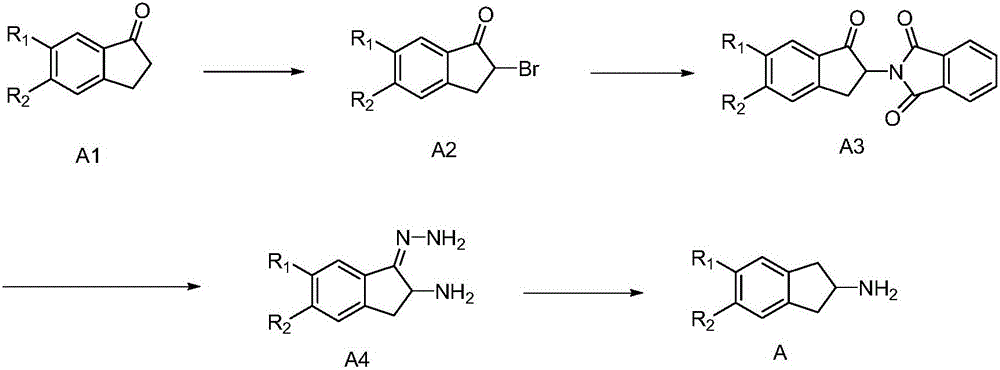
[0039] And, described synthetic method comprises the following steps:
[0040] (1) Using compound A1 as a starting material, carry out a bromination reaction to obtain intermediate A2;
[0041] (2) The intermediate A2 is prepared by the Gabriel reaction to obtain the intermediate A3;
[0042] (3) adding hydrazine hydrate to the intermediate A3, performing a hydrolysis reaction to generate hydrazone simultaneously, and obtaining the intermediate A4;
[0043] (4) The intermediate A4 is subjected to a hydrolysis reaction to obtain the target product 2-aminoindane derivative A;
[0044] Among them, R 1 and R 2 Each independently selected from: H, C1-C30 alkanyl, C3-C30 cycloalkyl, C2-C30 alkenyl, C3-C30 cycloalkenyl, C2-C30 alkynyl, C1- C30 alkoxy group, C7-C30 aromatic hydrocarbon group, C6-C30 aromatic hydrocarbon group, C4-C30 heterocyclic aromatic hydrocarbon group.
[0045] In a preferred embodiment, the synthesis method comprises the following steps:
[0046] (1) Disso...
Embodiment 1
[0063] The synthesis of embodiment 1 compound 1
[0064]
[0065] (1) Synthesis of intermediate 12
[0066] Dissolve 96.1g of compound 11 (0.5mol) in 500ml of ethyl acetate at room temperature, and slowly add bromine 80g (0.5mol) dropwise below 10°C. React until complete, add dropwise 1000ml of water, quench the layering, wash the organic phase once with 1000ml saturated brine, dry over anhydrous sodium sulfate and concentrate to dryness to obtain 135.5g (yield 98%) of yellow oily liquid, which is the intermediate Body 12.
[0067] (2) Synthesis of intermediate 13
[0068] At room temperature, 135.5g of intermediate 12 (0.5mol) and 101.87g of phthalimide potassium salt (0.55mol) were added to 1355ml of DMF, and stirred overnight (about 20 hours) at room temperature, the reaction solution It was dark brown, and TLC monitored the reaction until it was complete. After the reaction was complete, the reaction solution was poured into 6775ml of water, and a large amount of sol...
Embodiment 2
[0074] The synthesis of embodiment 2 compound 2
[0075]
[0076] (1) Synthesis of intermediate 22
[0077] Dissolve 81.1g of compound 21 (0.5mol) in 500ml of ethyl acetate at room temperature, keep below 10°C, slowly add 80g (0.5mol) of bromine dropwise, keep stirring at 20°C for 30 minutes, monitor by TLC After the reaction is complete, 1000ml of water is added dropwise to quench the layering, the organic phase is washed once with 1000ml of saturated brine, dried over anhydrous sodium sulfate and concentrated to dryness to obtain 120.54g of yellow oily liquid (99% yield), which is the intermediate Body 22.
[0078] (2) Synthesis of intermediate 23
[0079] At room temperature, 120.54g of intermediate 22 (0.5mol) and 101.87g of phthalimide potassium salt (0.55mol) were added to 1355ml of DMF, and stirred and reacted at room temperature for 2 hours. Brown, TLC monitors the reaction until complete, after the reaction is complete, the reaction solution is poured into 6775m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com