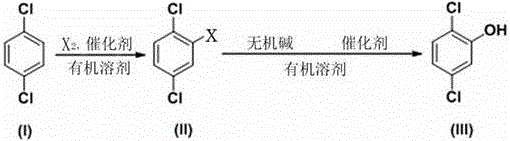
Synthesizing process of 2, 5-dichlorophenol
A technology for dichlorophenol and synthesis process, which is applied in the field of synthesis process of 2,5-dichlorophenol, can solve the problems of high industrialized production cost, environmental pollution, equipment corrosion, etc., and achieves reduction of the three-waste disposal amount and production cost. , the effect of short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of synthetic technique of 2,5-dichlorophenol, comprises the following steps:
[0036] A. Halogenation
[0037] Dissolve 1,4-dichlorobenzene in an organic solvent, add catalyst, add bromine at 30°C, react for 10 hours, remove residual bromine in the system, separate the organic phase, and concentrate and rectify the organic phase to obtain 2,5 -Dichlorobromobenzene; the molar ratio of bromine and 1,4-dichlorobenzene is 0.5:1, 2,5-dichlorobromobenzene conversion rate is 92.5%;
[0038] B. Hydrolysis
[0039] Dissolve the 2,5-dichlorobromobenzene obtained in step A in an organic solvent, add a copper salt catalyst, an inorganic base and water, react for 1 hour at a temperature of 150°C and a pressure of 1 MPa, and then pass the reaction liquid through alkali After washing, acidifying, extracting, and precipitation treatment, 2,5-dichlorophenol is obtained; the molar ratio of the inorganic base to 2,5-dichlorobromobenzene is 2:1, and the water and 2,5- The molar r...
Embodiment 2
[0042] A kind of synthetic technique of 2,5-dichlorophenol, comprises the following steps:
[0043] A. Halogenation
[0044] Dissolve 1,4-dichlorobenzene in an organic solvent, add catalyst, add bromine at 50°C, react for 6 hours, remove residual bromine, separate the organic phase, concentrate and rectify the organic phase to obtain 2,5- Dichlorobromobenzene; the molar ratio of bromine to 1,4-dichlorobenzene is 0.7:1, and the conversion rate of 2,5-dichlorobromobenzene is 95.3%;
[0045] B. Hydrolysis
[0046] Dissolve the 2,5-dichlorobromobenzene obtained in step A in an organic solvent, add a copper salt catalyst, an inorganic base and water, react for 3 hours at a temperature of 210°C and a pressure of 2 MPa, and then pass the reaction solution through alkali After washing, acidifying, extracting, and solvent removal, 2,5-dichlorophenol is obtained; the molar ratio of the inorganic base to 2,5-dichlorobromobenzene is 5:1, and the water and 2,5- The molar ratio of dichlo...
Embodiment 3
[0050] A kind of synthetic technique of 2,5-dichlorophenol, comprises the following steps:
[0051] A. Halogenation
[0052] Dissolve 1,4-dichlorobenzene in an organic solvent. After adding the catalyst, add iodine at 40°C. After reacting for 9 hours, remove the residual iodine, and separate the organic phase. The organic phase is concentrated and rectified to obtain 2,5- Dichloroiodobenzene; the molar ratio of the iodine to 1,4-dichlorobenzene is 0.6:1, and the conversion rate of 2,5-dichloroiodobenzene is 93.8%;
[0053] B. Hydrolysis
[0054] Dissolve the 2,5-dichlorobromobenzene obtained in step A in an organic solvent, add a copper salt catalyst and an inorganic base, react for 3 hours at a temperature of 180°C and a pressure of 1.5 MPa, and then wash the reaction solution with alkali , acidification, extraction, precipitation treatment to obtain 2,5-dichlorophenol; the molar ratio of the inorganic base to 2,5-dichloroiodobenzene is 4:1; 2,5-dichlorophenol hydrolysis yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com