Blood plasma virus inactivation device and method
A virus inactivation and plasma technology, applied in blood transfusion devices, suction devices, etc., can solve the problems of personal risk, high cost, methylene blue residue, etc., to overcome plasma virus inactivation, improve blood transfusion safety, and avoid safety. effect of risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
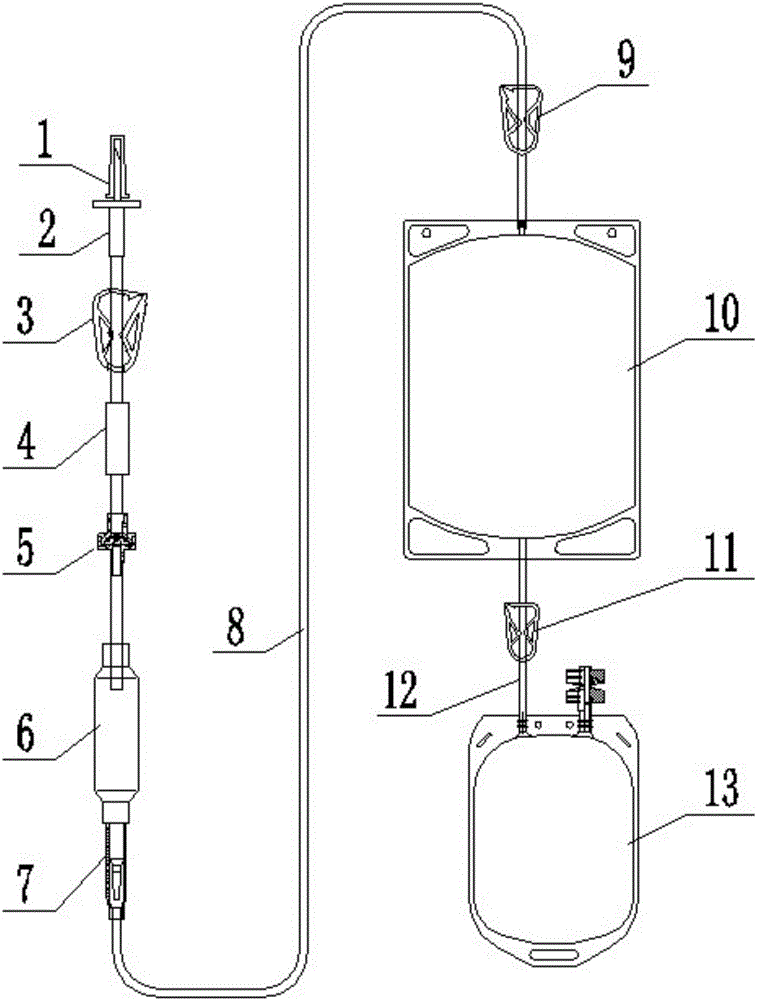
[0030] Such as figure 1 The shown plasma virus inactivation device mainly includes a dropping funnel 6, an illumination bag 10, a collection bag 13 and a puncture feeding part with a hollow inner cavity, and the described puncture feeding part is an elastic cylinder with a hollow inner cavity Body 4, the elastic cylinder 4 can be made of silica gel or TPE. One end of the elastic cylinder 4 communicates with the puncture device 2 through a pipeline connection, and the other end communicates with one end of the dropping funnel 6 through a pipeline connection, and the protective sleeve 1 is sealed and sleeved on the puncture device 2 to ensure the hygiene and Safety. A first liquid stop clip 3 is provided on the connecting pipeline between the piercer 2 and the elastic cylinder 4 , and a one-way valve 5 is arranged on the connecting pipeline between the elastic cylinder 4 and one end of the dropping funnel 6 . The other end of the dropping funnel 6 is connected to the inlet end...
Embodiment approach 2
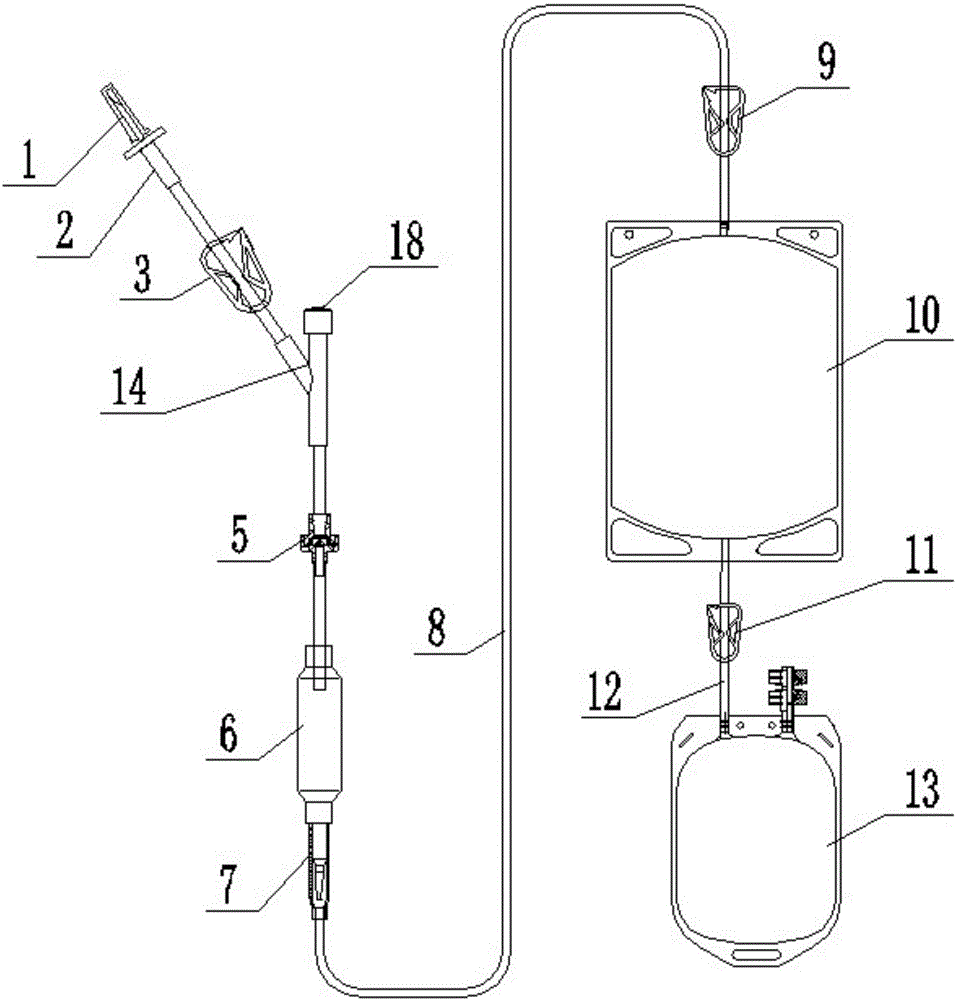
[0032] Such as figure 2 Compared with Embodiment 1, the plasma virus inactivation device shown uses a Y-shaped tee 14 instead of the elastic cylinder 4 . The Y-shaped tee 14 has 3 open ends, two of which are connected to the puncturer 2 and the drip funnel 6 through pipeline connections, and the other open end is sealed with a puncture cap 18 as a puncture dosing terminal, and other structures are the same as those in Embodiment 1.
[0033] The puncture cap 18 can be made of elastic materials such as silica gel or TPE, so that when the plasma virus inactivation operation is performed, the virus inactivation preparation can be injected into the dropping funnel 6 by directly puncturing the puncture cap 18, After the puncture and dosing is completed, the puncture cap 18 maintains a sealed state due to its elastic recovery, thereby avoiding the risk of contamination of the puncture and dosing.
Embodiment approach 3
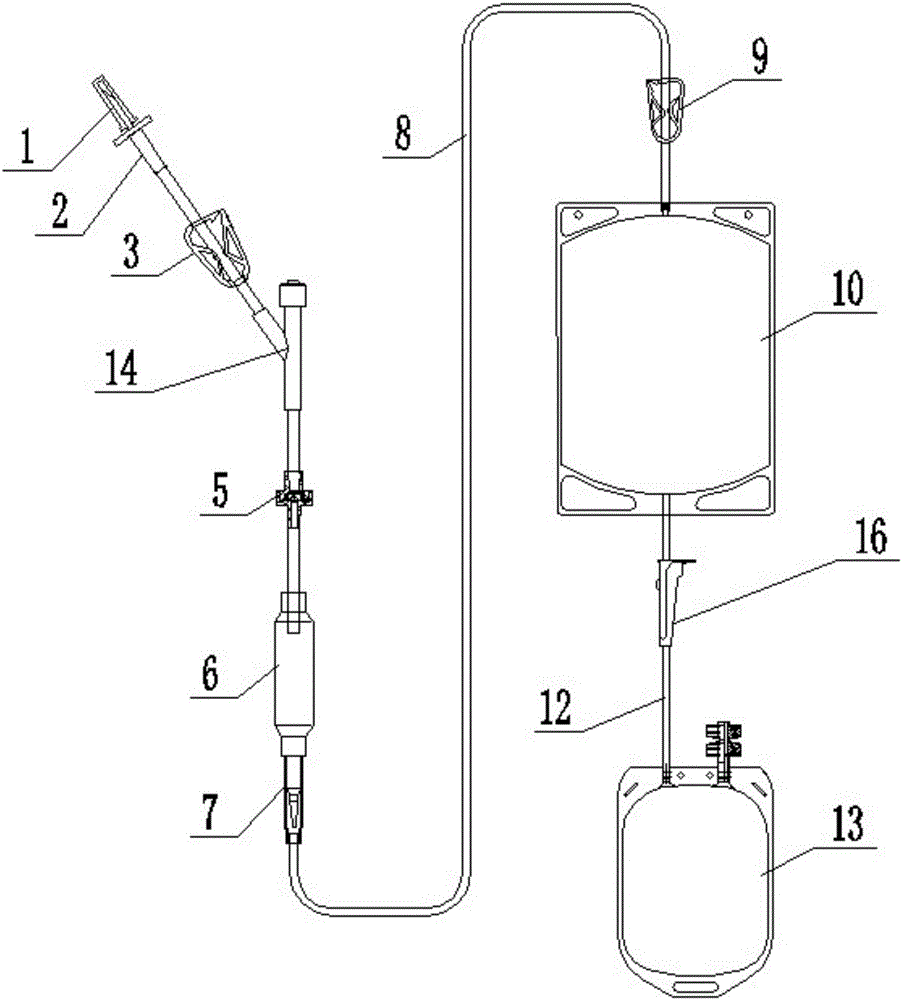
[0035] Such as image 3 Compared with Embodiment 2, the plasma virus inactivation device shown uses a flow regulator 16 instead of the third liquid stop clamp 11 as the pipeline cut-off device on the second conduit 12 , and the other structures are the same as Embodiment 2. By adjusting the roller on the flow regulator 16 to squeeze the second conduit 12, not only can the on-off of the second conduit 12 be controlled, but also the fluid flow in the second conduit 12 can be adjusted conveniently.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com