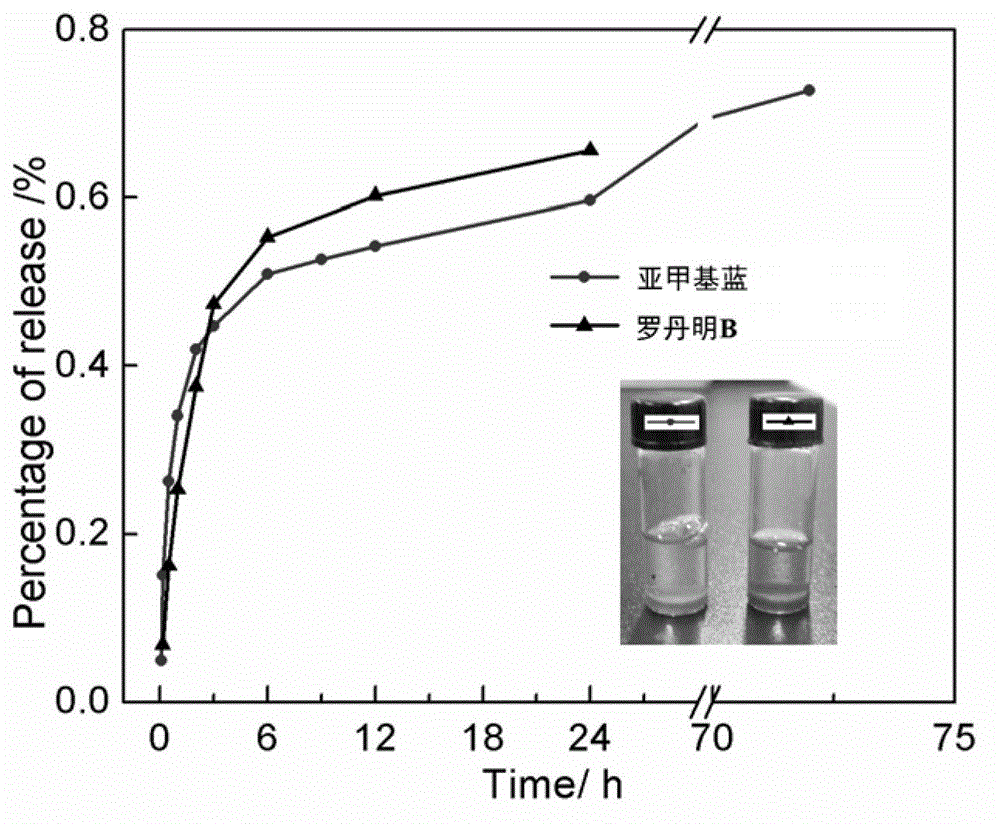
Multi-response supramolecular hydrogel factor, hydrogel and preparation method thereof
A supramolecular hydrogel and hydrogel technology are applied in the field of hydrogel and its preparation, multi-responsive supramolecular hydrogel factor, which can solve the problems of limited application and achieve good anti-tumor and anti-inflammatory effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Weigh 1.84 g of Fc-COOH (8 mM), dissolve it in 250 ml of anhydrous dichloromethane, add 1.20 g of HOBt and 3.36 g of HBTU (8.8 mM each) under ice cooling. About 6 mL of triethylamine was slowly added dropwise. The reaction was stirred under an ice bath for 40 minutes to 1 hour. Thin layer chromatography (TLC) spot plate followed the reaction. After the reaction is complete, use saturated NaCO 3 , 5% HCl and distilled water were extracted once each. The extract was concentrated, purified by column chromatography, and dried in vacuo. 2.60 g of dark red compound (1) was obtained.
[0052] Weigh 1.6 g of compound (1), dissolve it in 50 mL of anhydrous dichloromethane, and add 0.86 g of H-Phe-OMe·HCl. Then 2 mL of triethylamine was added dropwise, and the reaction was carried out at room temperature for about 10 hours. TLC spot plate followed the reaction. After the reaction is complete, use saturated NaCO 3 , 5% HCl and distilled water were extracted once each. The...
Embodiment 2
[0056] Fc-COOH, HOBt and HBTU with a molar ratio of 1:1.2:1.2 were dissolved in anhydrous dichloromethane, and triethylamine was slowly added dropwise under ice cooling to pH 8.0-9.0. The reaction was stirred for 40 minutes to 1 hour. Thin layer chromatography (TLC) spot plate followed the reaction. After the reaction is complete, use saturated NaCO 3 , 5% HCl and distilled water were extracted once each. The extract was concentrated, purified by column chromatography, and dried in vacuo. The dark red compound (1) was obtained.
[0057] Compound (1) and H-Phe-OMe·HCl with a molar ratio of 1:1.2 were dissolved in anhydrous dichloromethane, then triethylamine was added dropwise to pH 8.0-9.0, and the reaction was stirred at room temperature for about 10 hours. TLC spot plate followed the reaction. After the reaction is complete, use saturated NaCO 3 , 5% HCl and water were extracted once each. The extract was concentrated, purified by column chromatography, and dried in v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com