Trimetazidine hydrochloride sustained release tablets and preparation method thereof
A technology of trimetazidine hydrochloride and sustained-release tablets, which is applied in the fields of pharmaceutical formulations, cardiovascular system diseases, drug combinations, etc., and can solve problems such as short maintenance time of effective blood drug concentration, large side effects, and inability to exert stable drug effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
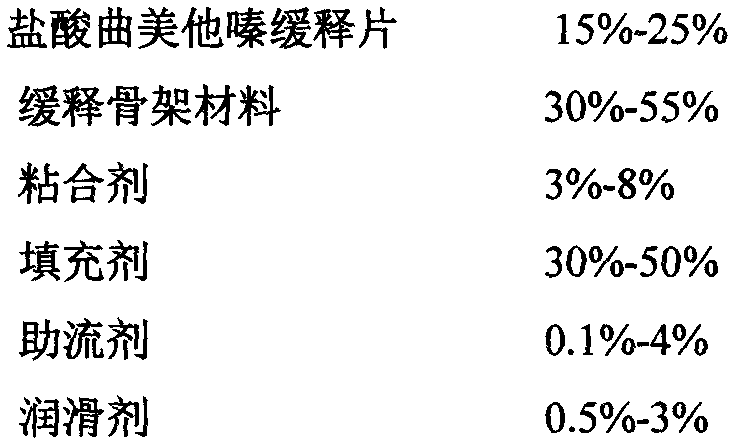
[0016] The following formula, according to the above-mentioned preparation method, is made into 4000 trimetazidine hydrochloride sustained-release tablets, and each sustained-release tablet finally obtained weighs about 200mg:
[0017]
[0018]
Embodiment 2
[0020] The following formula, according to the above-mentioned preparation method, is made into 4000 trimetazidine hydrochloride sustained-release tablets, and each sustained-release tablet finally obtained weighs about 200mg:
[0021]
[0022] Adopt the device in the second appendix XC dissolution test method of Chinese Pharmacopoeia 2010 edition, according to the test method of appendix XD release rate, get each slow-release tablet in embodiment 1-2 respectively, take water 500ml as dissolution medium, rotating speed 75 rev / min, operate according to the above method, take the corresponding solution at 1, 2, 3, 4, and 8 respectively, filter, accurately pipette 5ml of filtrate into a 10ml volumetric flask with a pipette gun, and use 0.1mol / L Dilute the sulfuric acid solution to the mark, shake well, measure the absorbance at 232nm by ultraviolet-visible spectrophotometry, and calculate the release amount.
[0023]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com