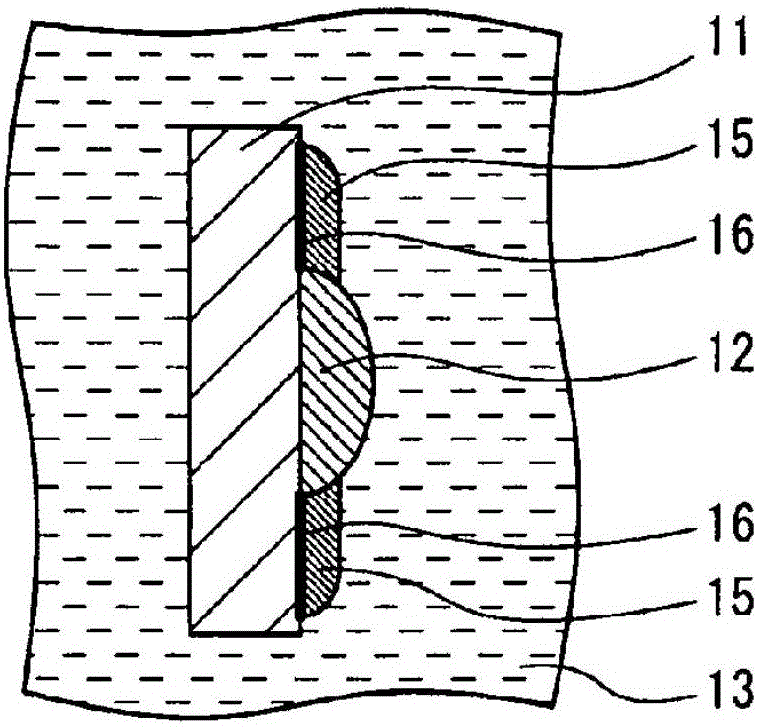
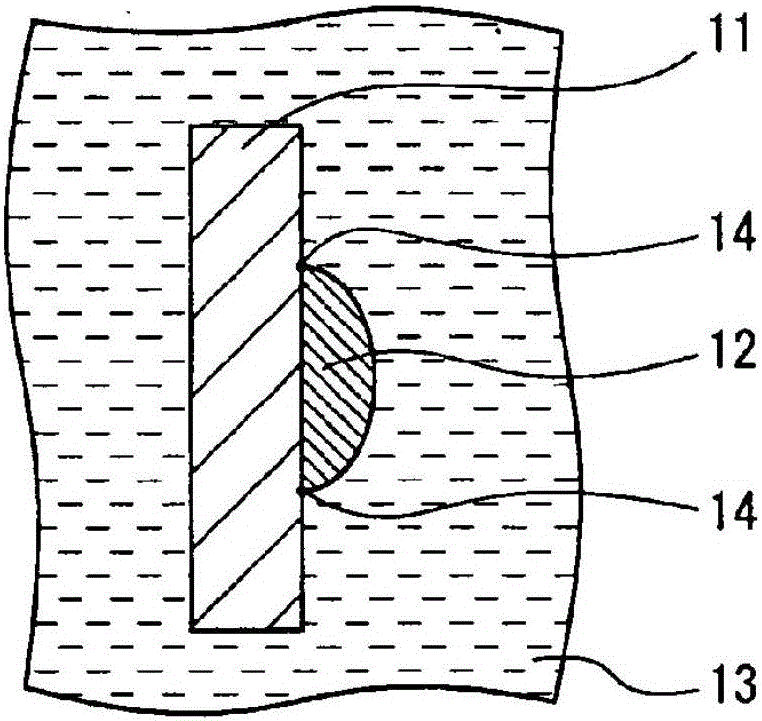
Secondary battery
A secondary battery and electrolyte technology, which is applied to secondary batteries, lithium batteries, battery electrodes, etc., can solve the problems of short charge and discharge life and poor energy efficiency, and achieve the effect of long charge and discharge life and good energy efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
[0099] In the cell configuration shown in Table 1 below, except for the type and amount of the anion acceptor added to the electrolytic solution, six identical lithium batteries were produced as Examples 1 to 6, respectively. The composition of the electrolytic solution is as described in Table 2 below. In addition, in the following Table 1, "coin-shaped cell size 2032 type" represents the coin-shaped cell (lithium battery) with a diameter of 20 mm and a height of 3.2 mm. "SUS316" represents one of the JIS standards of stainless steel. Other abbreviations described in the following Tables 1 and 2 are as described in the columns of the following Table 1.
[0100] [Table 1]
[0101]
[0102] AB: Acetylene black
[0103] PVDF: polyvinylidene fluoride
[0105] DMC: Dimethyl Carbonate
[0106] [Table 2]
[0107]
[0108] It should be noted that the anion acceptor used in this example and the fluoride ion F are shown in the following Tabl...
Embodiment 7
[0143] A lithium secondary battery was produced as described below. Assembly was carried out in a glove box replaced with argon in the same manner as in Examples 1 to 6.
[0144] Coin unit: diameter 20mm, thickness 3.2mm
[0145] Positive plate: FeF 270wt% (about 0.055g), acetylene black (carbon) 25wt%, PTFE (polytetrafluoroethylene), trade name "Teflon (registered trademark)" 5wt%, diameter 13mm, thickness about 0.5mm
[0146] Negative electrode: Li metal sheet, diameter 13mm, thickness 0.2mm
[0147] Electrolyte: 1M LiPF 6 EC / DMC (volume ratio 1:1, density about 1.3g / cm 3 ) by adding 0.2M of TPFPB
[0148] Separator: Polypropylene microporous membrane
[0149] FeF in coin cell 2 Molar ratio to TPFPB: about 1:0.14
[0150] [Charge and discharge characteristics (lifetime provision)]
[0151] In the battery of Example 7, charging and discharging were repeated five times under the following conditions.
[0152] Charge and discharge conditions: 1.0V-4.5V
[0153] Charg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com