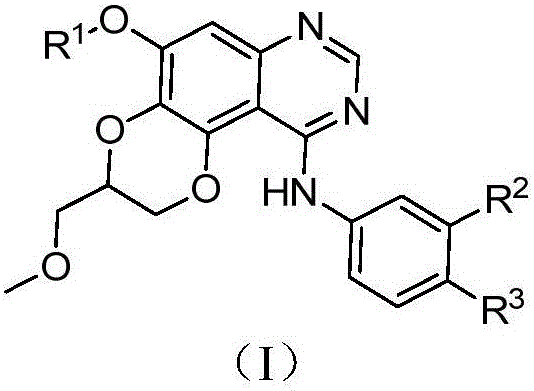
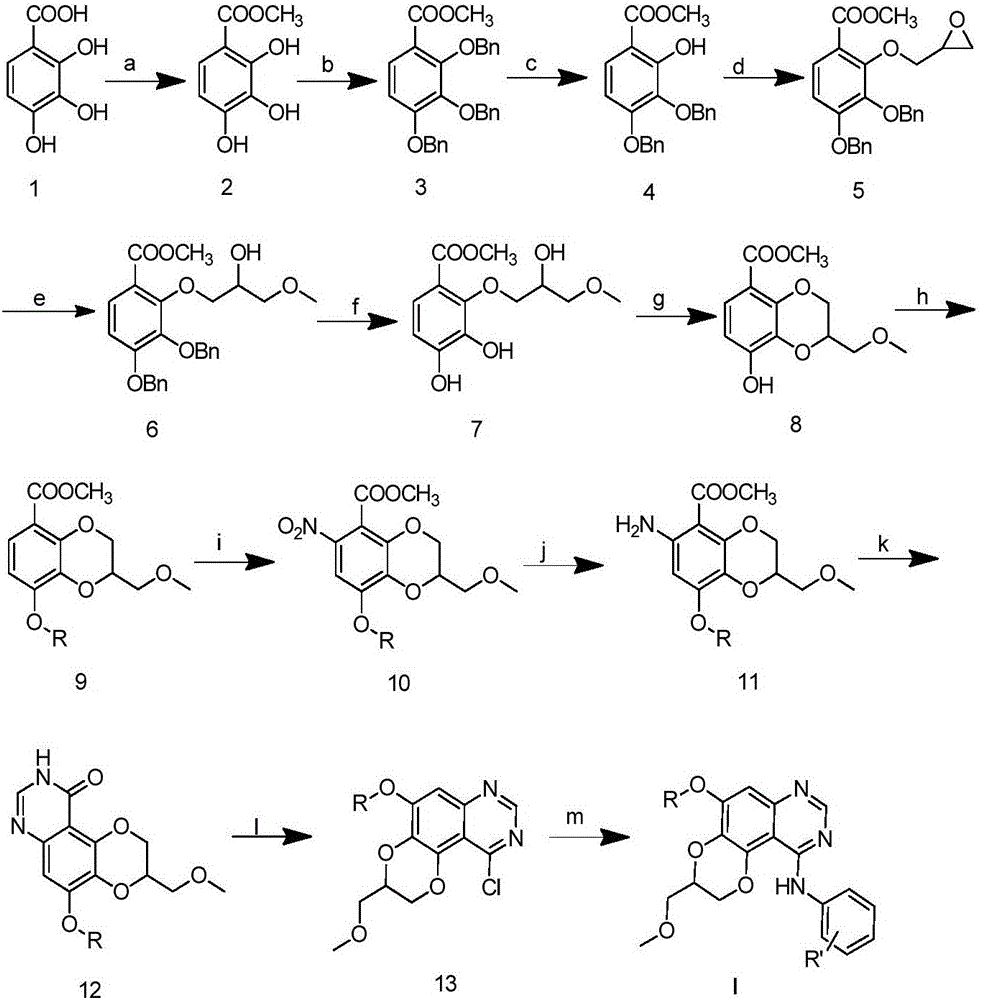
Dioxanoquinazoline amine compound and preparation method thereof, and application of dioxanoquinazoline amine compound as epidermal growth factor receptor inhibitor
A compound and dioxane technology, applied in organic chemistry, drug combination, pharmaceutical formulation, etc., can solve the problem of limited survival time extension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] N-(3-chloro-4-fluorophenyl)-5-(2-methoxyethoxy)-3-(methoxymethyl)-2,3-dihydro[1,4]dioxin Preparation of alkano[2,3-f]quinazolin-10-amine (I-1)
[0054] Step 1) Preparation of 2,3,4-trihydroxybenzoic acid methyl ester
[0055]
[0056] At room temperature, add 34 grams (200 mmol) of 2,3,4-trihydroxybenzoic acid and 40 grams (400 mmol) of potassium bicarbonate, and 300 mL of N,N-dimethylformamide into the reactor and stir slowly for 0.5 hours, then add 42 g ( 300 mmol) methyl iodide, reacted for 12 hours, poured into 1500 mL of water, filtered with suction, washed with water, and dried to obtain 35 g of white solid with a yield of 95%. 1 H NMR (400MHz, CDCl 3 )δ11.01(s,1H),7.38(d,J=8.8Hz,1H),6.53(d,J=8.8Hz,1H),5.80(s,1H),5.47(s,1H),3.94( s,3H).ESI-MS m / z:185[M+H] + .
[0057] Step 2) Preparation of 2,3,4-tribenzyloxymethylbenzoate
[0058]
[0059] Mix 18.5 grams (100 mmol) of methyl 2,3,4-trihydroxybenzoate, 42.0 grams (300 mmol) of potassium carbonate and 15...
Embodiment 2
[0094] N-(3-fluorophenyl)-5-(2-methoxyethoxy)-3-(methoxymethyl)-2,3-dihydro[1,4]dioxano[2 , 3-f] the preparation of quinazoline-10-amine (I-2)
[0095]
[0096] Steps 1) to 12) are the same as in Example 1;
[0097] 10-Chloro-5-(2-methoxyethyl)-3-(methoxymethyl)-2,3-dihydro[1,4]dioxane[2,3-f]quinazole Add morphine (100mg, 0.29mmol) into a 25mL round-bottomed flask, add 10mL of isopropanol and stir, add 3-fluoroaniline (38mg, 0.35mmol), heat to reflux for 2h, cool, filter the precipitated crystals and wash with a small amount of isopropanol The crystals were washed and dried to obtain 69 mg of white solid with a yield of 58%. Melting point: 231.6-232.2°C. 1 H NMR (500MHz, CD 3 OD+CDCl 3 )δ8.58(s,1H),7.63(dt,J=10.3,2.2Hz,1H),7.48–7.37(m,2H),7.11(s,1H),7.09–7.01(m,1H),4.83 (dd,J=11.4,2.2Hz,1H),4.55–4.50(m,1H),4.44(dd,J=11.4,7.5Hz,1H),4.38(t,J=4.4Hz,2H),3.88( dd,J=7.2,3.7Hz,2H),3.82(qd,J=10.7,4.9Hz,2H),3.47(d,J=1.5Hz,6H). 13 CNMR (125MHz, CD 3 OD+CDCl 3 ( d,J=9.1Hz),...
Embodiment 3
[0099] N-(3-chlorophenyl)-5-(2-methoxyethoxy)-3-(methoxymethyl)-2,3-dihydro[1,4]dioxano[2 ,3-f] the preparation of quinazoline-10-amine (I-3)
[0100]
[0101] Steps 1) to 12) are the same as in Example 1;
[0102] 10-Chloro-5-(2-methoxyethyl)-3-(methoxymethyl)-2,3-dihydro[1,4]dioxane[2,3-f]quinazole Add morphine (100mg, 0.29mmol) into a 25mL round-bottomed flask, add 10mL of isopropanol and stir, add 3-chloroaniline (44mg, 0.35mmol), heat to reflux for 2h, cool, filter the precipitated crystals and wash with a small amount of isopropanol The crystals were washed and dried to obtain 77 mg of white solid with a yield of 62%. Melting point: 233.3-234.7°C. 1 H NMR (500MHz, CD 3 OD+CDCl 3 )δ8.59(s,1H),7.82(s,1H),7.56(d,J=8.0Hz,1H),7.43(t,J=8.1Hz,1H),7.33(d,J=8.0Hz, 1H),7.14(s,1H),4.84(dd,J=11.3,1.7Hz,1H),4.54(d,J=5.0Hz,1H),4.45(dd,J=11.3,7.5Hz,1H), 4.40(t, J=4.3Hz, 2H), 3.92–3.87(m, 2H), 3.83(qd, J=10.8, 4.8Hz, 2H), 3.49(d, J=0.9Hz, 6H). 13 C NMR (125MHz, CD 3 OD+CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com