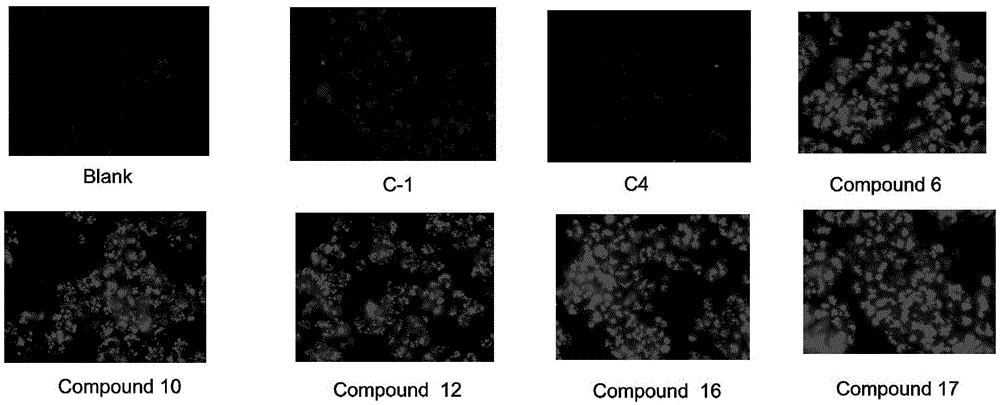
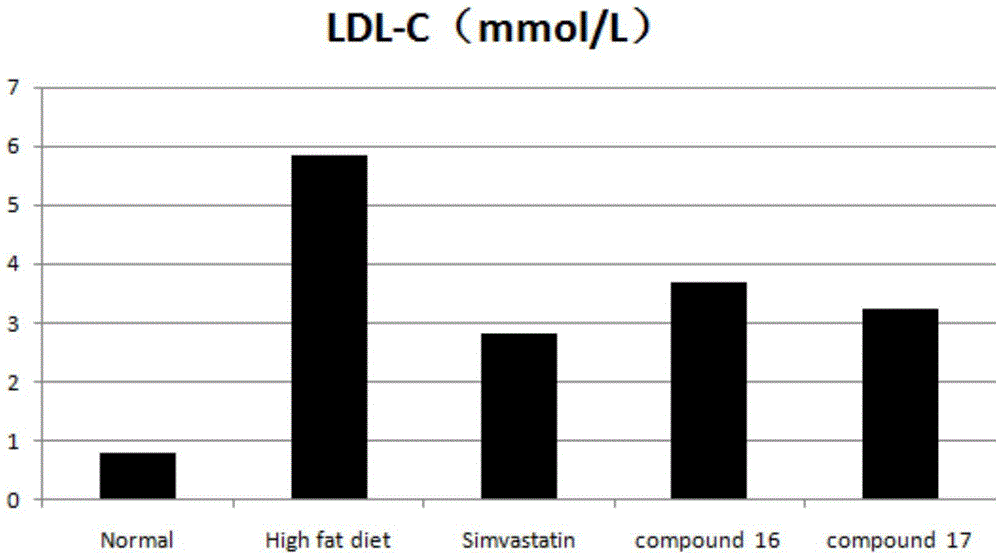
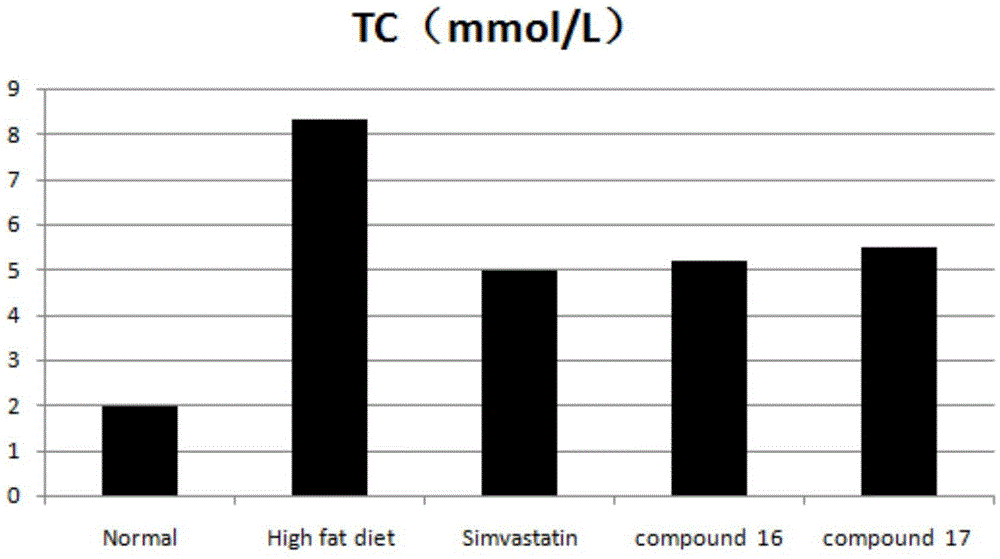
Tetrahydroisoquinoline derivative and application thereof
A technology of salts and compounds, applied in the field of tetrahydroisoquinoline derivatives and their applications, can solve problems such as adverse reactions, only injection preparations, gallstones, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0204] The general method of the present invention is further explained below, the compound of the present invention can be prepared by methods known in the art, and the preparation method of the preferred compound of the present invention is used as an example to illustrate in detail below, but the preparation method of the compound of the present invention is not limited thereto .
[0205] The preparation of the general formula compound (V) disclosed by the present invention is mainly prepared according to the following scheme:
[0206]
[0207] The above preparation route is described as follows: X1 and X2 are the starting materials of this scheme, which can be obtained from commercially available products, or prepared according to methods reported in literature. Under certain reaction conditions, X1 and X2 can obtain compound X3 through a certain amide connection method. The intermediate X3 is reacted under the action of a dehydrating agent to obtain X4. Further connec...
Embodiment 1
[0226]
[0227] 1A (508μL, 3.01mmol) and 1B (500mg, 3.01mmol) were added to a 25mL round bottom flask, placed in an oil bath, heated to 150°C, melted into a liquid, and stirred for 2.5h. Cool to room temperature, add ethyl acetate, grind the solid, filter with suction, wash with ethyl acetate, and dry to obtain off-white solid 1C (765 mg, yield 77.3%)
[0228]Add 1C (500mg, 1.52mmol), toluene (3mL), and phosphorus oxychloride (750μL) into a 50mL round bottom flask, stir in an oil bath at 110°C for 2h, cool to room temperature, add saturated sodium bicarbonate in an ice bath The aqueous solution was quenched, extracted with ethyl acetate, washed with water, dried over anhydrous sodium sulfate, and spin-dried to obtain an oil. The oil was dissolved in methanol (15mL), stirred at room temperature, sodium borohydride (115mg, 3.04mmol) was added in batches, after the addition was completed, stirred at room temperature for 20min, the reaction solution was spin-dried, the residue ...
Embodiment 2
[0231]
[0232] 2A (100mg, 0.319mmol) was added to a 50mL round bottom flask, dissolved in dichloromethane (10mL), triethylamine (67μL, 0.479mmol), 2B (69μL, 0.639mmol) were added, stirred at room temperature for 3h, and the reaction solution was vortexed Dry, the residue was extracted with water and ethyl acetate, the organic phase was dried with anhydrous sodium sulfate, and spin-dried, the obtained residue was separated and purified by preparative TLC (petroleum ether: ethyl acetate 2:1) to obtain a colorless paste compound 2 ( 121 mg, yield 90.3%). 1 HNMR (400MHz, CDCl 3 )δ7.07–7.00(m,2H),6.85–6.78(m,2H),6.58(s,1H),6.12(s,1H),4.86(t,J=6.9Hz,1H),3.84(s ,3H),3.78(s,3H),3.72(ddd,J=13.6,6.3,2.2Hz,1H),3.62(s,3H),3.38(ddd,J=13.8,11.4,4.6Hz,1H), 3.17(dd,J=13.6,6.4Hz,1H),3.06–2.93(m,2H),2.66–2.55(m,7H); MS(ESI)m / z[M+H] + :421.12
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com