Preparation method of semaglutide and intermediate of semaglutide
A technology of samoglutide and intermediates, applied in the field of medicinal chemistry, can solve the problems of increasing the difficulty of coupling, affecting the purity of crude peptides, affecting the yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0091] The invention discloses a preparation method of samaglutide and an intermediate thereof. Those skilled in the art can refer to the content herein to obtain samaglutide. In particular, it should be noted that all similar substitutions and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The preparation method and application of the present invention have been described through preferred embodiments, and relevant personnel can obviously make changes or appropriate changes and combinations to the preparation method and application herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0092] All the reagents and raw materials used in the preparation method of samaglutide and its intermediate provided by the present invention can be purchased from the market.
[0093] See Table 1 for the Chinese-English com...
Embodiment 1 18
[0098] Embodiment 1 octadecanedioic acid monomethyl ester is synthesized
[0099] Weigh 87g (0.5mol) of octadecanedioic acid into a three-necked flask, add 500mL of DCM, stir to dissolve, add 16g (0.5mol) of methanol, dropwise add 0.05g of concentrated sulfuric acid, stir until reflux, and react for 4 hours. TLC monitors that the reaction is completed, the reaction solution is cooled, and 2% Na is added 2 CO 3 (Mass volume percentage) The aqueous solution was extracted twice with 100 mL, the aqueous layer was extracted twice with 100 mL DCM, the organic phases were combined, and dried over anhydrous sodium sulfate. The filtrate was concentrated under reduced pressure to obtain 69.5 g of the product.
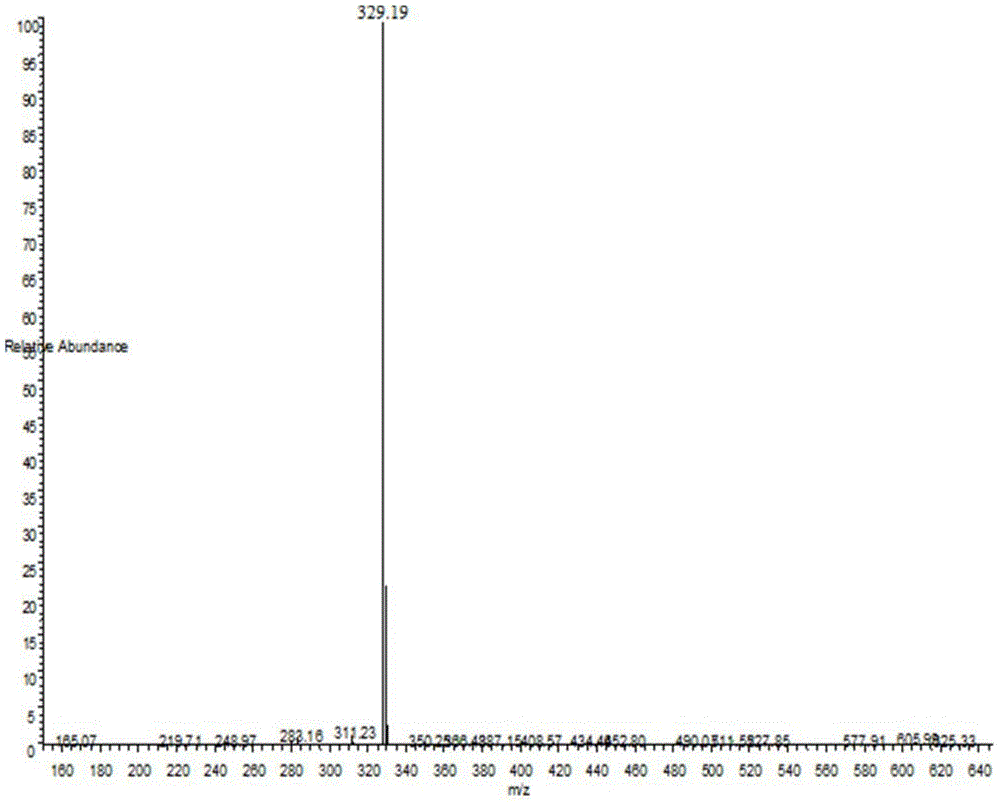
[0100] Adopt mass spectrometry and proton nuclear magnetic resonance spectrum to detect the resulting product, the mass spectrogram of gained is shown in figure 1 , as can be seen from the figure, MS, m / z329.19 (M+H) + ; Gained proton nuclear magnetic resonance spectrum data ...
Embodiment 2
[0101] Embodiment 2 degree of substitution is the synthesis of Fmoc-Lys(Alloc)-2CTC resin of 0.60mmol / g
[0102] Weigh 20 g of 2-CTC resin (purchased from Tianjin Nankai Synthetic Technology Co., Ltd.) with a degree of substitution of 1.0 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, then take 9.05 gFmoc-Lys(Alloc)-OH was dissolved in DMF, activated by adding 6.65mL DIEA in an ice-water bath, and then added to the above-mentioned reaction column filled with resin. After 2 hours of reaction, 10mL of anhydrous methanol was added to block for 1 hour. Wash 3 times with DMF and 3 times with DCM, then block with anhydrous methanol for 30 minutes, shrink and dry with methanol to obtain Fmoc-Lys(Alloc)-2CTC resin, and the detected substitution degree is 0.605mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com