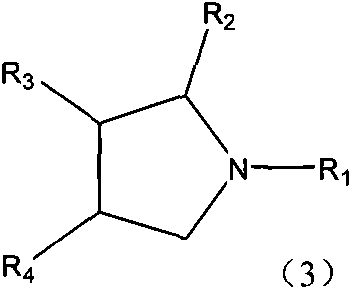
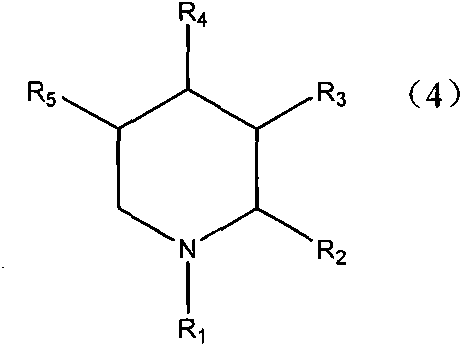
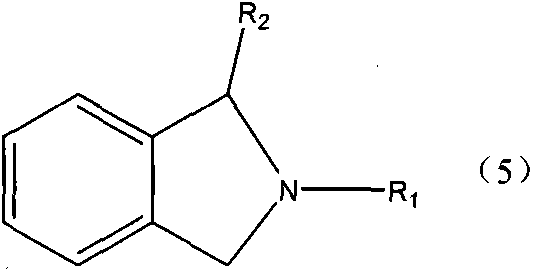
Production method of single nitrogenous heterocyclic ring-containing compound
A nitrogen heterocyclic compound and a technology for heterocyclic compounds are applied in the production field of single nitrogen heterocyclic compounds, which can solve the problems of poor safety performance, low product yield, rare raw materials, etc. High selectivity, process safety and environmentally friendly results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Take phthalide (another name o-hydroxymethylbenzoic acid lactone, content 99%) and dissolve it in water at 85°C according to the mass ratio of 1:1, and enter a continuous tubular reactor R101 (inner diameter 80mm, length 6m ) enters at 1m below the center, the internal catalyst is alumina containing 1.5wt% CaO, and the feed rate is 10L / h. Liquid ammonia enters from the lower part of the R101 reactor and reacts in reverse with the phthalide aqueous solution. The feed rate is 2.5L / h, and the converted molar ratio is about 2.2. The crude product (the amount of phthalide contained therein is 0.2wt%) enters the secondary flash tank, and the liquid is taken out in the lower part of the second flash tank, and subsequently obtains a compound containing 89 wt% indolinone (type I compound), wherein water Content 10wt%, phthalide 0.6wt%, free amine 2400ppm, the above-mentioned mixture containing indolinone is mixed with hot hydrogen by a metering pump and then enters the vaporizer...
Embodiment 2
[0045] γ-butyrolactone (content 99.5%) and liquid ammonia enter a continuous tubular reactor R101 (inner diameter 120mm, length 15m) according to the molar ratio of 1: 1.8 through metering pump, reaction temperature 260-270 ℃, reaction pressure 8.0MPa, The feed rate is 60L / h. The crude product (the gamma-butyrolactone amount contained therein is 0.4wt%) in the top of the outlet R101 enters the three-stage flash tank, and the liquid is taken out at the third-stage flash tank bottom, and subsequently obtains a product containing 95wt% 2-pyrrolidone (I type compound), wherein the water content is 4.5wt%, butyrolactone 0.4wt%, free amine 600ppm, the above-mentioned mixture containing 2-pyrrolidone is mixed with hot hydrogen by a metering pump and then enters the vaporizer, and the liquid feed rate is 5L / h, the hydrogen feed rate is 20NM3 / h, the equivalent hydrogen to 2-pyrrolidone molar ratio is 14.5, and after being overheated by a superheater (control temperature 230-250°C), it...
Embodiment 3
[0047] δ-valerolactone (content 99.8%) and monoethylamine aqueous solution (monoethylamine content 70wt%) enter a continuous stirred tank reactor R101 (2NM 3), the reaction temperature is 150°C, the reaction pressure is 2.5MPa, the catalyst is in powder form, the content is 2wt% of magnesium oxide, 45% of aluminum oxide, 53wt% of silicon oxide, and the amount of feed and output is 400L / h. The crude product (wherein containing δ-valerolactone amount is 1.5wt%) of the top of outlet R101 enters the first-stage flash tank, and the liquid is taken out at the bottom of the flash tank, and then obtained containing 93wt% 2-ethyl-piperidone ( Type I compound), wherein water content 1.2wt%, δ-valerolactone 0.8wt%, free amine 200ppm, the above-mentioned mixture containing 2-ethyl-piperidone is mixed with hot hydrogen by a metering pump and enters the vaporizer , the liquid feed rate is 10L / h, the hydrogen feed rate is 60NM 3 / h, the equivalent hydrogen to 2-ethyl-piperidone molar ratio ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com