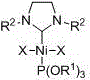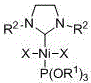Method for preparing arylboronic acid neopentyl glycol ester
A technology of neopentyl glycol diboronic acid ester and neopentyl glycol ester, which is applied in the field of preparing neopentyl glycol diboronic acid ester, and can solve the cross-coupling reaction between chlorinated aromatic hydrocarbons and neopentyl glycol diboronic acid ester that has not been seen and other issues, to achieve the effects of large-scale synthesis and application, high yield and easy product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment one: Ni[P(OR 1 ) 3 ][(R 2 NCH 2 CH 2 NR 2 )C]X 2 (R 1 =CH 2 CH 3 , R 2 =2,4,6-trimethylphenyl, X=Br) synthesis
[0020] The azacyclic carbene (R 2 NCH 2 CH 2 NR 2 )C (0.2464g, 0.8 mmol) was added to a THF solution of bis(triethylphosphite) nickel dibromide (0.4400 g, 0.8 mmol), reacted at room temperature for 2 hours, removed the solvent in vacuo, and washed with n-hexane The residue was extracted with toluene, the clear liquid was transferred and the solvent toluene was removed to obtain a red solid with a yield of 68%.
[0021] The product was subjected to elemental analysis, and the results are shown in Table 1:
[0022] Table 1 Elemental analysis results
[0023]
C:(%)
H:(%)
N:(%)
theoretical value
46.86
6.12
4.05
actual value
47.04
6.21
3.99
[0024] The product was characterized by NMR, and the results are as follows:
[0025] Dissolve the product in C 6 D. 6 Medium (a...
Embodiment 2
[0026] Embodiment two: Ni[P(OR 1 ) 3 ][(R 2 NCH 2 CH 2 NR 2 )C]X 2 (R 1 =CH 2 CH 3 , R 2 =2,6-diisopropylphenyl, X=Br) synthesis
[0027] The azacyclic carbene (R 2 NCH 2 CH 2 NR 2)C (0.3627 g, 0.93 mmol) was added to a tetrahydrofuran solution of di(triethylphosphite)nickel dibromide (0.5115 g, 0.93 mmol), reacted at room temperature for 2 hours, removed the solvent in vacuo, and washed with n-hexane Wash the residue, extract the residue with toluene, transfer the clear liquid and remove the solvent toluene to obtain red crystals with a yield of 77%.
[0028] Product is carried out elemental analysis, and the result is as shown in table 2:
[0029] Table 2 Elemental analysis
[0030]
C:(%)
H:(%)
N:(%)
theoretical value
51.06
7.01
3.61
actual value
51.33
7.19
3.49
[0031] The product was characterized by NMR, and the results are as follows:
[0032] Dissolve the product in C 6 D. 6 Medium (a...
Embodiment 3
[0033] Embodiment three: Ni[P(OR 1 ) 3 ][(R 2 NCH 2 CH 2 NR 2 )C]X 2 (R 1 =CH(CH 3 ) 2 , R 2 =2,6-diisopropylphenyl, X=Br) synthesis
[0034] The azacyclic carbene (R 2 NCH 2 CH 2 NR 2 )C (0.3627 g, 0.93 mmol) was added to a tetrahydrofuran solution of di(triisopropyl phosphite) nickel dibromide (0.5905 g, 0.93 mmol), and reacted at room temperature for 3 hours, and the solvent was removed in vacuo, and the Wash the residue with alkane, extract the residue with toluene, transfer the clear liquid and remove the solvent toluene to obtain red and black crystals with a yield of 70%.
[0035] Product is carried out elemental analysis, and the result is as shown in table 3:
[0036] Table 3 elemental analysis
[0037]
[0038] The product was characterized by NMR, and the results are as follows: the product was dissolved in C 6 D. 6 Medium (about 0.4mL), seal the tube, measure and characterize on a UnityInova-400 NMR instrument at room temperature: 1 HNM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com