Compounds from antrodia camphorata, method for preparing the same and use thereof
一种提取物、药物的技术,应用在牛樟芝提取物及其制备领域,能够解决抗癌效果所知甚少等问题,达到抑制多种癌细胞增生、抑制效果提升、抑制血管新生的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
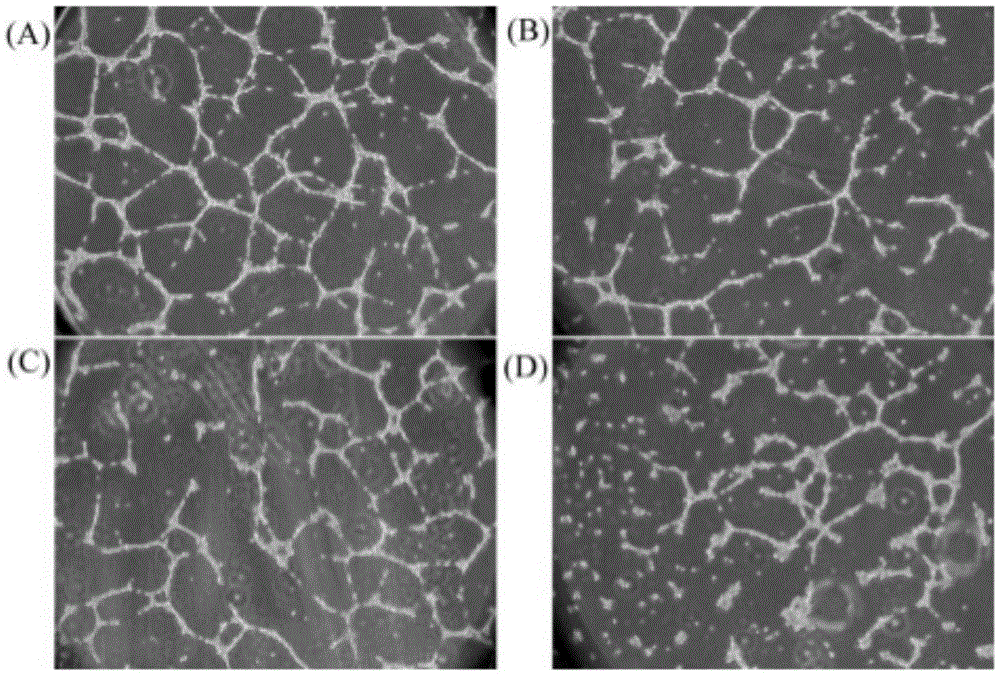
Image
Examples
Embodiment 1
[0057] The preparation of embodiment 1 Antrodia camphorata extract
[0058] The liquid fermented mycelium of Antrodia camphorata is refluxed twice with n-hexane, each time for 1-3 hours; after suction and filtration, the two n-hexane extracts are combined; the silica gel with 70-230 holes is taken (silicagel) (70-230mesh) and mycelium weight 1:1 (w / w) to fill the column, elute with n-Hexane / Ethylacetate (Ethylacetate) gradient, and the eluate is divided into fractions (fraction) F1 , F2 and F3, the gradients are 17-22%% Ethylacetate, 23-27% Ethylacetate and 28-33% Ethylacetate respectively, and F3 is divided into three sections F3-1-F3-3 according to the order of retention time.
[0059] Get F3-1 and use mobile phase as dichloromethane / acetone (CH 2 Cl 2 / Acetone) is separated by silica gel column chromatography (gradient elution from 50:1 to 20:1 in polarity), and the section of 40:1-15:1 is taken, and the normal phase HPLC (Medium pressure liquid chromatography) semi-prepa...
Embodiment 2
[0066] The chemical structure analysis of embodiment 2 Antrodia camphorata extract
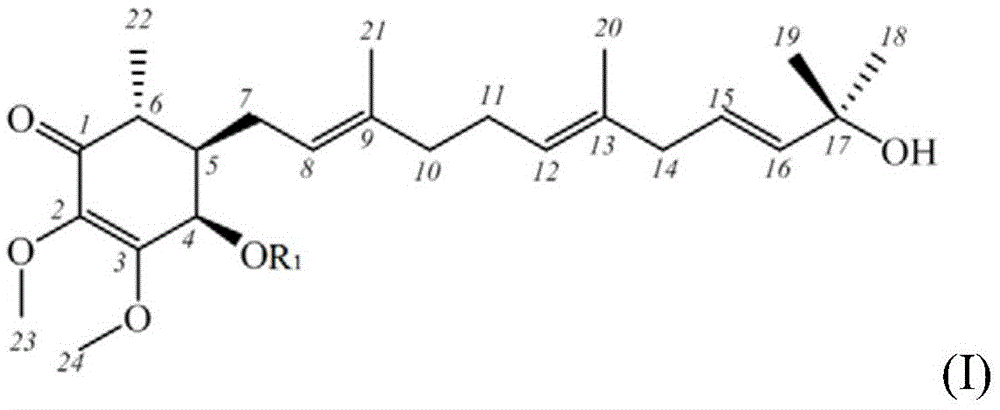
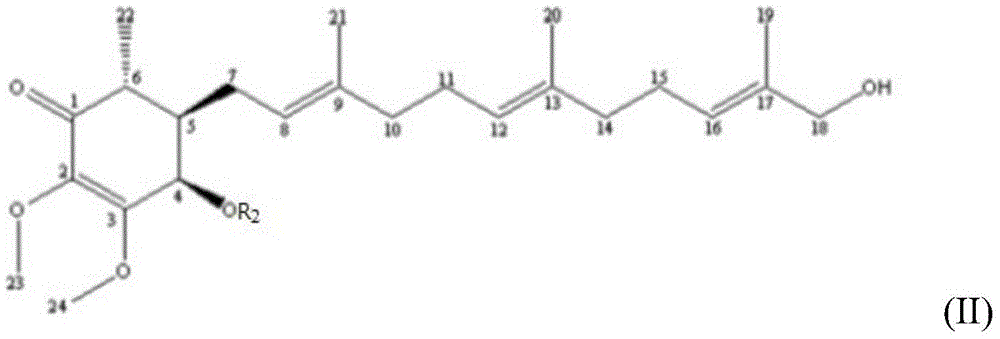
[0067] For the aforementioned purified Antrodia camphorata extract, its chemical structure was identified by spectroscopic analysis, including the use of 1D / 2 DNMR and mass spectrometry. The structural analysis results of the compound are as follows:
Embodiment 21
[0069]
[0070] EIMS, m / z 471.2701[M+Na]+; 1 HNMR (400MHz, CD 3 OD)δ5.77(1H,d,J=3.2Hz,H-4),5.18(1H,t,J=5.6Hz,H-12),5.16(1H,t,J=6.4Hz,H-8 ),5.11(1H,dt,J=1.6,8.8Hz,H-16),4.43(1H,q,J=6.8,H-15),4.00(3H,s,H-24),3.62(3H, s,H-23),2.53(1H,m,H-6),2.29(1H,m,H-7a),2.24(2H,m,H-14),2.11(2H,m,H-11) ,2.10(3H,s,-OAc),2.08(1H,m,H-10a),2.02(1H,m,H-7b),1.94(1H,m,H-10b),1.92(1H,m, H-5), 1.71(3H,d,J=1.2Hz,H-18),1.66(3H,d,J=1.2Hz,H-19),1.64(3H,s,H-20),1.58( 3H, s, H-21), 1.18 (3H, d, J=6.8Hz, H-22); 13 CNMR (100MHz, CD 3 OD)δ199.2(s,C-1),171.6(s,-COCH 3 ),160.7(s,C-3),138.9(s,C-2),138.8(s,C-9),134.9(s,C-17),132.9(s,C-13),129.6(d ,C-16),128.4(d,C-12),122.2(d,C-8),70.4(d,C-4),68.1(d,C-15),61.2(q,C-23) ,60.4(q,C-24),49.2(t,C-14),44.4(d,C-5),42.6(d,C-6),40.9(t,C-10),28.1(t, C-7), 27.6(t, C-11), 26.1(q, C-18), 21.0(q, -COCH 3 ), 18.5 (q, C-19), 16.8 (q, C-20), 16.5 (q, C-21), 13.3 (q, C-22).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com