Multifunctional fusion polypeptide and preparation method and application thereof
A fusion polypeptide, multi-functional technology, applied in the preparation method of peptide, fusion polypeptide, hybrid peptide and other directions, can solve the problems of single target, easy to produce drug resistance, lack of specific drugs for the treatment of pulmonary fibrosis, etc. The method is simple, the effect of increasing the activity of anti-pulmonary fibrosis and anti-pulmonary infection, and good application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation and inspection of the multifunctional polypeptide of the present invention
[0043] In this example, polypeptides I-VI were all synthesized by solid-phase synthesis, separated and purified by preparative HPLC, and the purity of the polypeptide was determined by analytical high-performance liquid chromatography.
[0044] Polypeptide I-VI solid-phase synthesis method uses solid-phase carrier Fmoc-wang-resin (Gill Biochemical Co., Ltd.) as the starting material, and then uses protected amino acids to inoculate dipeptide to nonacupeptide in sequence, and fully washes the peptide after the work is completed. , Peptide cutting, and post-treatment to obtain the crude product of the polypeptide; dissolve the crude product, purify twice with preparative high-performance liquid phase, concentrate and freeze-dry to obtain the pure product, and finally obtain the refined product of the polypeptide through the third purification. The method can not only ensure the synthe...
Embodiment 2
[0073] In this example, polypeptides I-VI were all synthesized by liquid phase synthesis, separated and purified by preparative HPLC, and the purity of the polypeptide was determined by analytical high performance liquid chromatography. The following are the synthesis steps of polypeptide I, and the synthesis steps of polypeptide II-VI are the same as those of polypeptide I.
[0074] Polypeptide I synthesis steps are as follows:
[0075] 1. According to the sequence of polypeptide I, in 10 mL of dichloromethane, 1 mg of the first amino acid Pro and 1 mg of the second amino acid D-Pyr are connected through an amide bond, and the inactive groups of the amino acids participating in the reaction are modified with Fmoc.
[0076] 2. Add 10 mL of ammonia water to the above reaction system to remove the Fmoc group.
[0077] 3. Repeat the reaction of the first step, add the third amino acid D-Cys according to the sequence of the synthesized polypeptide, and modify the inactive group o...
Embodiment 3
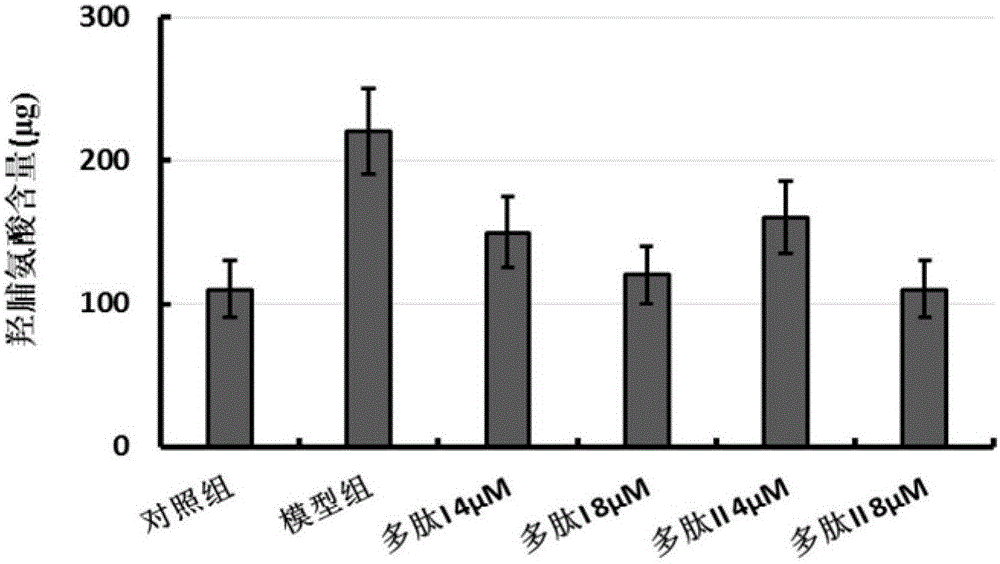
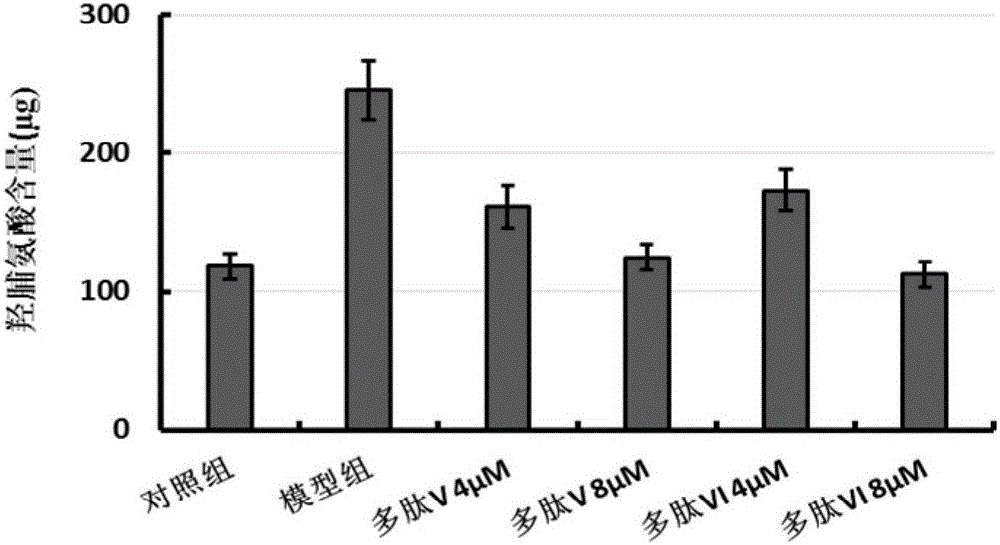
[0081] Establishment of In Vitro Pulmonary Fibrosis Model
[0082] Human non-small cell lung cancer cell A549 was cultured in DMEM medium containing 10% (volume fraction) fetal bovine serum, cultured in a 5% carbon dioxide incubator at 37°C, and the medium was changed every other day, depending on the cell density. Take A549 cells in the logarithmic growth phase, digest with 0.25% trypsin to make cell suspension, adjust the cell concentration to 1×10 9 pcs / L, and according to 4×10 5 piece / cm 2 Density seeded at 90cm 2 In the cell culture dish, they were randomly divided into two groups: (1) control group: cultured with DMEM containing 10% (volume fraction) fetal bovine serum; (2) model group: containing 10% (volume fraction) fetal bovine serum, DMEM culture of transforming growth factor (TGF-β1, final concentration 5 μg / L). Place them in an incubator at 37°C with a volume fraction of 5% carbon dioxide, change the medium every other day, and passage as scheduled depending o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com