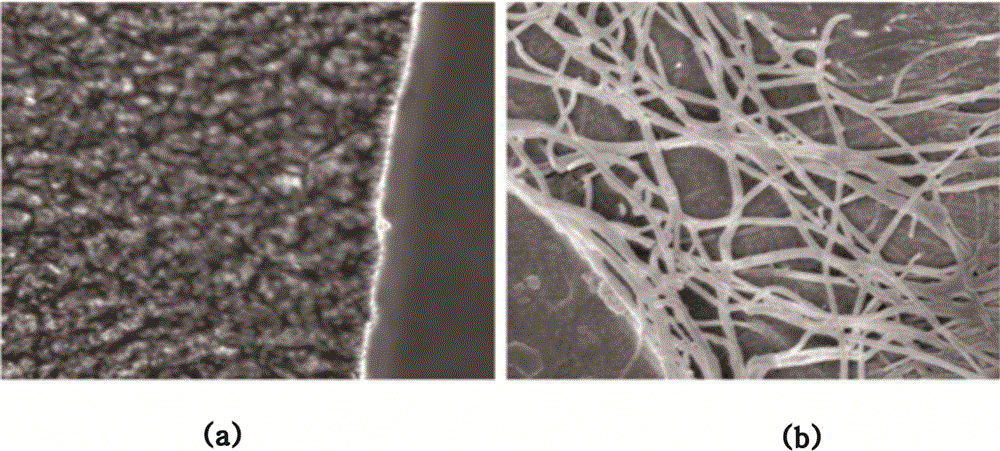
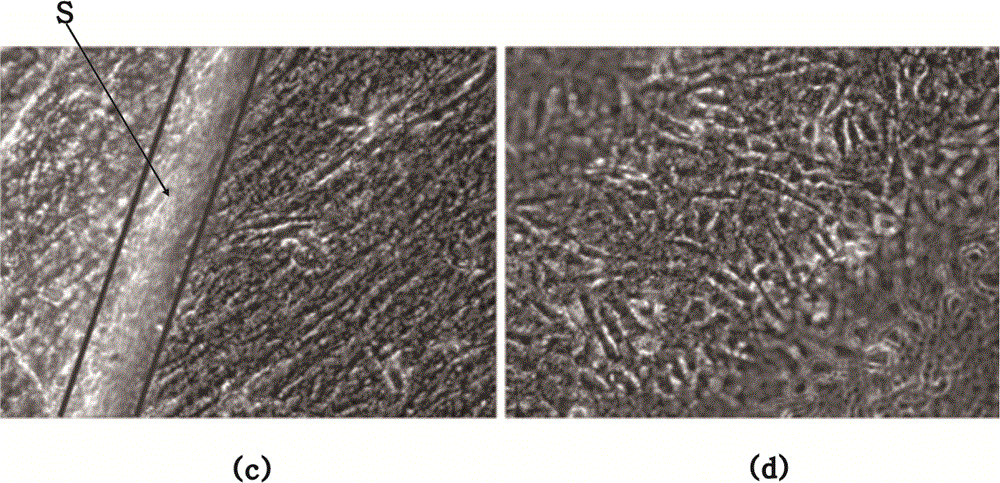
Chorion decellularizing liquid and decellularizing method
A chorionic and decellularized technology, which is applied in medical science, tissue regeneration, prostheses, etc., can solve the problems of easy curling and falling off, difficult attachment, slow wound healing, etc., to achieve commercial production, not easy to curl, and widely popularized Applied effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Step 1. Wash the placenta with 0.9% normal saline, and then peel off the chorion;
[0041] Step 2, put the chorion into the decellularized chorion preparation solution on a shaker, which includes 0.8% sodium dodecylsulfonate, 0.6% polyethylene glycol octylphenyl ether, 5% sodium chloride, 2% DNAse, 0.2% protease inhibitor, 0.8% trypsin, adjust the rotation speed to 200 rpm, shake at room temperature for 30 hours;
[0042] Step 3. Take out the chorion, wash it in pure water on a shaker, 200 rpm, shake at room temperature for 1 hour, repeat 8 times;
[0043] Step 4. Soak the cleaned chorion in 0.1 peracetic acid for 5 hours to sterilize it, and store it at 4°C for later use.
Embodiment 2
[0045] Step 1. Wash the placenta with 0.9% normal saline, and then peel off the chorion;
[0046] Step 2, put the chorion into the decellularized chorion preparation solution on the shaker, which includes 1% sodium dodecylsulfonate, 0.5% polyethylene glycol octylphenyl ether, 4% sodium chloride, 1.5% DNAse, 0.4% protease inhibitor, 0.6% trypsin, adjust the rotation speed to 160 rpm, shake at room temperature for 36 hours;
[0047] Step 3. Take out the chorion, wash it in pure water on a shaker, 160 rpm, shake at room temperature for 1 hour, repeat 7 times;
[0048] Step 4. Soak the cleaned chorion in 0.1 peracetic acid for 4 hours to sterilize it, and store it at 4°C for later use.
Embodiment 3
[0050] Step 1. Wash the placenta with 0.9% normal saline, and then peel off the chorion;
[0051] Step 2, put the chorion into the decellularized chorion preparation solution on the shaker, which includes 1% sodium dodecylsulfonate, 2% polyethylene glycol octylphenyl ether, 6% sodium chloride, 2.5% DNAse, 0.5% protease inhibitor, 1% trypsin, adjust the rotation speed to 180 rpm, shake at room temperature for 24 hours;
[0052] Step 3. Take out the chorion, wash it in pure water on a shaker, 180 rpm, shake at room temperature for 1 hour, repeat 8 times;
[0053] Step 4. Soak the cleaned chorion in 0.3 peracetic acid for 3 hours to sterilize it, and store it at 4°C for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com