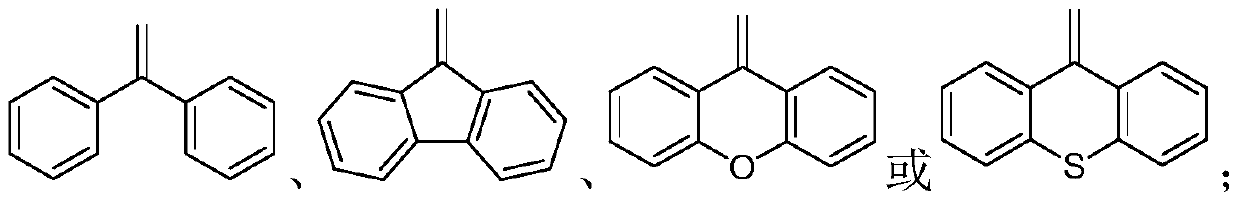
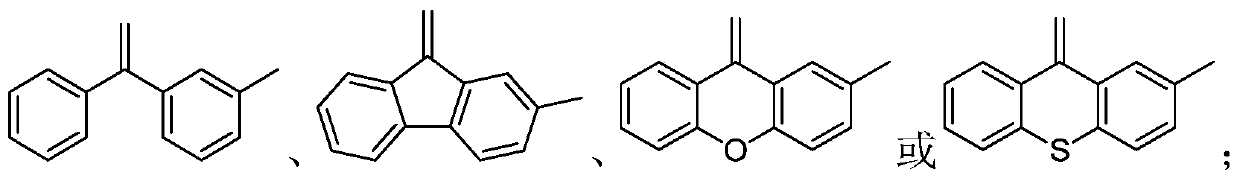
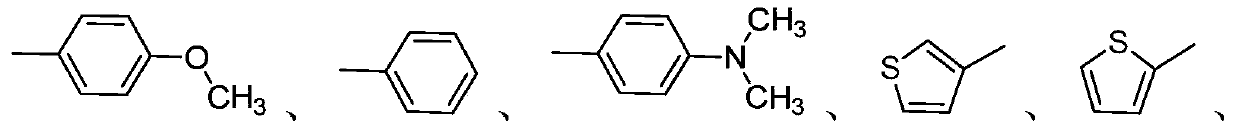
Aggregation-induced emission compound as well as preparation method and application thereof
An aggregation-induced luminescence and compound technology, which is applied in the field of aggregation-induced luminescence compounds and their preparation, can solve problems such as the reduction of fluorescence quantum yield, and achieve the effects of easy long-term storage, improved synthesis efficiency, and stable fluorescence quantum yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation method of the aggregation-induced luminescence compound comprises the following steps:
[0039] (1) 1eq of the compound of formula VII or 0.5eq of the compound of formula VIII 2eq~3eq or 0.5eq~0.15eq of Pd(PPh 3 ) 4 And 0.5eq~0.15eq of phase transfer catalyst, mixed in a solvent with a pH value of 9~12, fully reacted under an inert gas atmosphere at 70°C~100°C, to obtain a compound of formula V or compound of formula VI Wherein, the phase transfer catalyst is tetrabutylammonium bisulfate, tetrabutylammonium bromide or benzyltriethylammonium chloride; the solvent is that the mass ratio of organic solvent to water is 2:1~4:1 A mixed solvent, the organic solvent is toluene, tetrahydrofuran or dioxane.
[0040] (2) 1eq of a compound of formula V or 0.5eq of a compound of formula VI, 4eq to 6eq of carbon tetrabromide and 8eq to 12eq of triphenylphosphine are fully reacted in an organic solvent at 80°C to 150°C to obtain formula III compound or compo...
Embodiment 1
[0044] a kind of like figure 1 The synthetic route diagram of the dendrimer with fluorene as the central core shown comprises the following steps:
[0045] Synthesis of a central core with a bromine functional group: 3.604g (20mmol) of fluorenone, 13.270g (40mmol) of carbon tetrabromide and 17.530g (80mmol) of triphenylphosphine were placed in 100mL of anhydrous dichloromethane, stirred and refluxed 24h. The crude product was separated by a column to obtain 4.7 g of yellow solids, and a central core with a bromine functional group was obtained, and the chemical structural formula was
[0046] (1) Synthesis of the first generation of dendrimers (I-A2)
[0047] 0.68g (2mmol) 9-dibromoethylene-fluorene, 1.53g (5mmol) borate of fluorenone, 0.23g (0.2mmol) tetrakis (triphenylphosphine) palladium, 68mg (0.2mmol) tetrabutylsulfuric acid Ammonium hydrogen and 0.83 g (6 mmol) of potassium carbonate were placed in a mixed solution of 40 mL of toluene and 20 mL of distilled water, h...
Embodiment 2
[0052] Repeat Example 1 with the same steps as described, the difference is that the p-methoxyphenylboronic acid in the step (3) is replaced with the borate of fluorenone to obtain the product I-A2A4, the chemical structural formula is
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com