Benzimidazole quinoline derivative and preparation method and application
A technology of benzimidazoquinoline and benzimidazole, applied in the field of analytical chemistry, can solve the problems of slow sample detection, unsuitable for on-site detection or large-scale popularization and application, complex sample pretreatment process, etc., and achieves easy operation and detection method. Diverse and complex effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (A) Preparation of 2-methyl-8-(2-benzimidazolyl) quinoline (1)
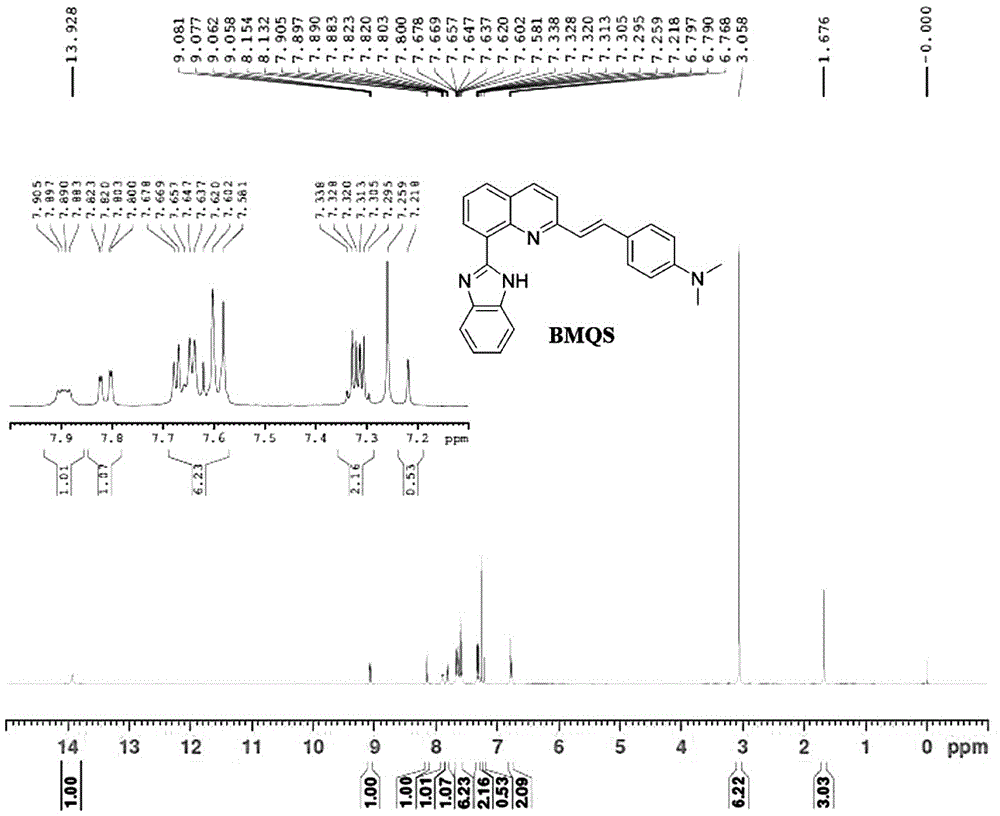
[0035] After mixing 2-methylquinoline-8-carboxylic acid (4.8620g, 26mol), double equivalent o-phenylenediamine (3.0888, 28.6mol) and polyphosphoric acid (26.3562, 78mol), stir and reflux for more than 6 hours, cool Finally, add the reaction solution into a beaker filled with a large amount of water, adjust the pH to neutral with ammonia water, stir, solids precipitate out, filter with suction, recrystallize with 60% ethanol, the yield is 70.54%, and the melting point is 173.5-174.3°C. Nuclear Magnetic Resonance Spectrum Proton 1H NMR (400MHz, CDCl3): δ: 13.72(br,1H),9.11(d,1H),8.16(d,1H),7.86(d,2H),7.65(t,2H),7.39 (d, 1H), 7.29-7.31 (m, 2H), 2.91 (s, 3H).
[0036] (B) 2-(4-N,N-Dimethylstyryl)-8-(1H-benzimidazole)quinoline (copper ion fluorescent probe)
[0037] Add 2-methyl-8-benzimidazolylquinoline (0.8750g, 3.38mmol), N,N-dimethylbenzaldehyde (2.0210g, 13.5mmol) into a 50mL two-necked bottle, and under...
Embodiment 2
[0039]Preparation procedure of the test solution: in a 10mL sample bottle, add 5.0mL double distilled water, then add 0.1mol / L Cu2+ standard solution (20μL, 20eq), then add 5.0mL acetonitrile, mix well (pH=6.40) ; Finally, add 100 μL of 1×10-3 probe BMQS in acetonitrile solution and mix again. After standing for 10 minutes, the ultraviolet absorption and fluorescence emission were measured at 420nm as the excitation wavelength. The above operation, without adding metal ion solution, is the preparation of blank test solution. Measure UV absorption and fluorescence emission.
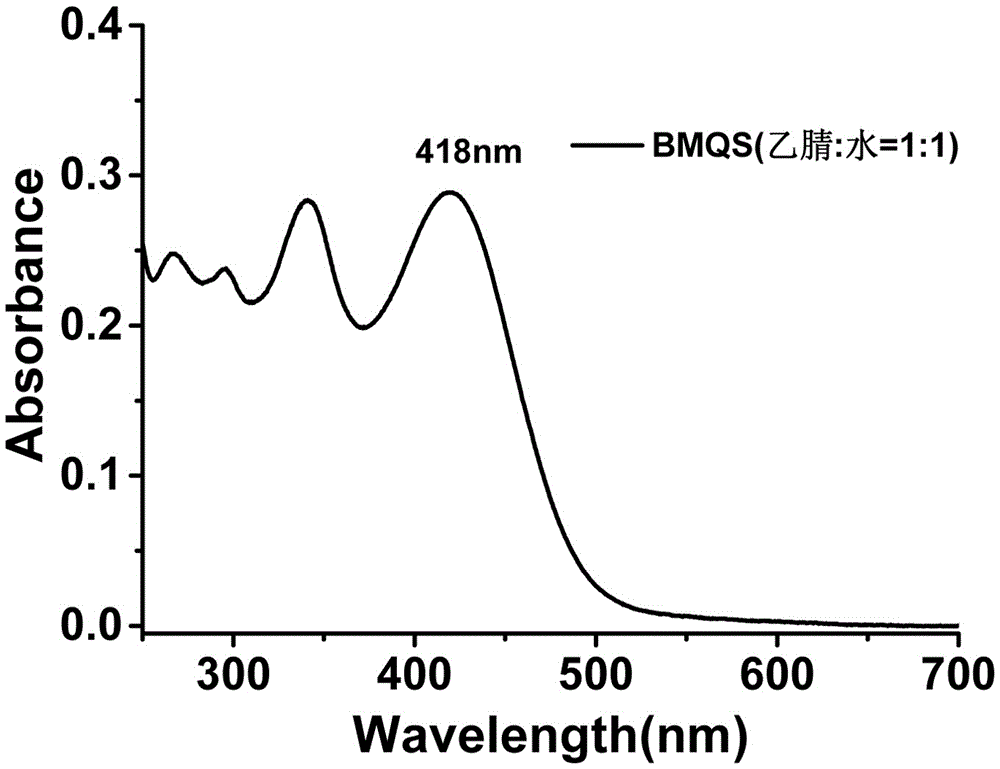
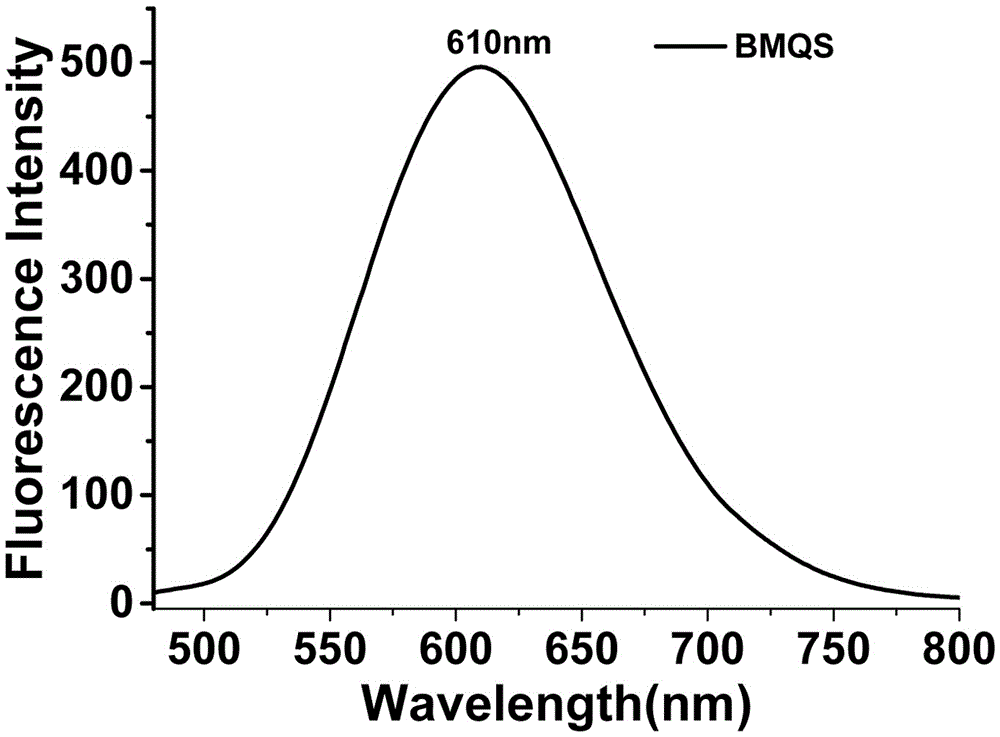
[0040] UV spectrum and fluorescence spectrum: The blank test solution of probe BMQS has strong absorption at 418nm and no absorption at 550nm; when copper ions exist, the absorption at 418nm is significantly weakened, and the absorption at 550nm is significantly enhanced, see Figure 7 . The blank test solution of probe BMQS has a strong fluorescence emission peak at 610nm. After adding copper ions, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com