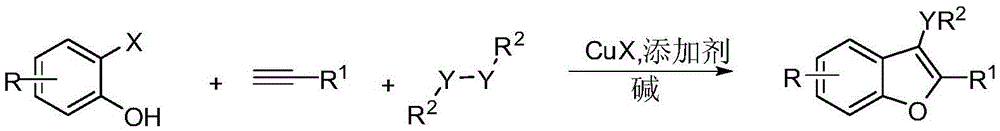Preparation method of polysubstituted benzofuran derivative
A technology for benzofuran and derivatives, which is applied in the field of preparation of multi-substituted benzofuran derivatives, can solve problems such as pollution and complicated preparation methods, and achieve the effect of strong operability and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
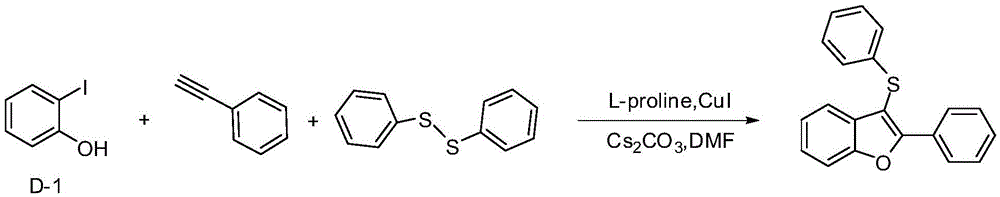
Embodiment 1
[0018] Example 12-Phenyl-3-(phenylthio)benzofuran
[0019]
[0020] In a 25mL three-neck flask, add D-1 (220mg, 1mmol), and then add Cs 2 CO 3 (652mg, 2mmol), diphenyl disulfide (109mg, 0.5mmol), L-proline (34.5mg, 0.3mmol), cuprous iodide (19mg, 0.1mmol), phenylacetylene (102mg, 1mmol) , 5mLDMSO, under the protection of nitrogen, react in an oil bath at 90°C for 20h, add 5mL water after cooling, extract with 5mL ethyl acetate each time, after repeating four times, the extract is washed with saturated brine, anhydrous sulfuric acid After drying over sodium, the filtered liquid was distilled under reduced pressure, and the distillate was separated through a silica gel column (petroleum ether as the eluent) to obtain 248.4 mg of a yellow viscous liquid with a yield of 82%.
[0021] 1 HNMR (500MHz, CDCl 3 )δ7.67–7.58(m,2H),7.58–7.49(m,2H),7.38(t,J=7.4Hz,2H),7.35–7.27(m,5H),7.21(dd,J=12.4, 4.6Hz, 2H), 7.11(t, J=7.3Hz, 1H).
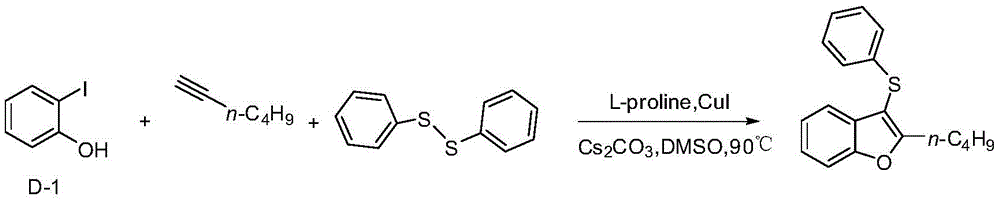
Embodiment 2
[0022] Example 22-n-Butyl-3-(phenylthio)benzofuran
[0023]
[0024] In a 25mL three-neck flask, add D-1 (220mg, 1mmol), and then add Cs 2 CO 3 (652mg, 2mmol), diphenyl disulfide (109mg, 0.5mmol), L-proline (34.5mg, 0.3mmol), cuprous iodide (19mg, 0.1mmol), 1-hexyne (82mg, 1mmol), 5mLDMSO, under the protection of nitrogen, react in an oil bath at 90°C for 20h, add 5mL water after cooling, extract with 5mL ethyl acetate each time, after repeating four times, the extract is washed with saturated brine, no After drying with sodium sulfate, the filtered liquid was distilled under reduced pressure, and the distillate was separated through a silica gel column (petroleum ether as the eluent) to obtain 203.4 mg of a yellow viscous liquid with a yield of 72%.
[0025] 1 HNMR (500MHz, CDCl 3 )δ7.61(s,1H),7.44(s,1H),7.20(s,1H),7.19-7.16(m,3H),7.09-7.01(m,3H),2.94(s,2H),1.68 (s,2H),1.37(s,2H),0.92(s,3H).
Embodiment 3
[0026] Example 32-(4-methoxyphenyl)-3-(phenylthio)benzofuran
[0027]
[0028] In a 25mL three-neck flask, D-1 (220mg, 1mmol), then add K 2 CO 3 (276mg, 2mmol), diphenyl disulfide (109mg, 0.5mmol), L-proline (34.5mg, 0.3mmol), cuprous chloride (9.9mg, 0.1mmol), p-methoxyphenylacetylene (132mg, 1mmol), 5mLDMSO, under the protection of nitrogen, react in an oil bath at 100°C for 20h, add 5mL of water after cooling, extract with 5mL of ethyl acetate each time, after repeating four times, extract the solution with saturated saline After washing, drying over anhydrous sodium sulfate, the filtered liquid was distilled under reduced pressure, and the distillate was separated through a silica gel column (petroleum ether as the eluent) to obtain 282.6 mg of a light yellow viscous liquid with a yield of 85%.
[0029] 1HNMR (500MHz, CDCl3) δ7.54–7.46(m,4H),7.36(t,J=7.5Hz,2H),7.32–7.27(m,2H),7.19(t,J=7.7Hz,2H), 7.14(s,1H),7.10(t,J=7.3Hz,1H),6.79(d,J=8.8Hz,2H),3.75(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com