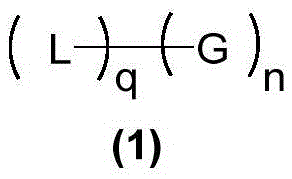Dynamic polymer material
A polymer material, dynamic technology, applied in the direction of coating, etc., can solve the problems affecting the use of materials, the reduction of thiol content, the use environment and application limitations of dynamic polymer materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0178] In the twelfth embodiment of the present invention, a dynamic polymer material includes a product obtained by the mutual reaction of the following components:
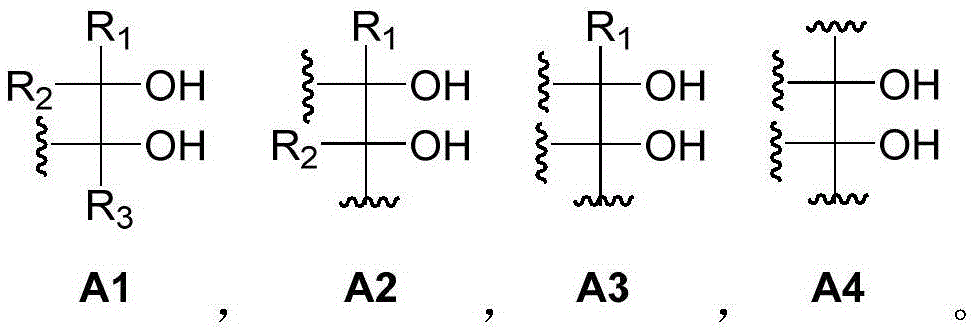
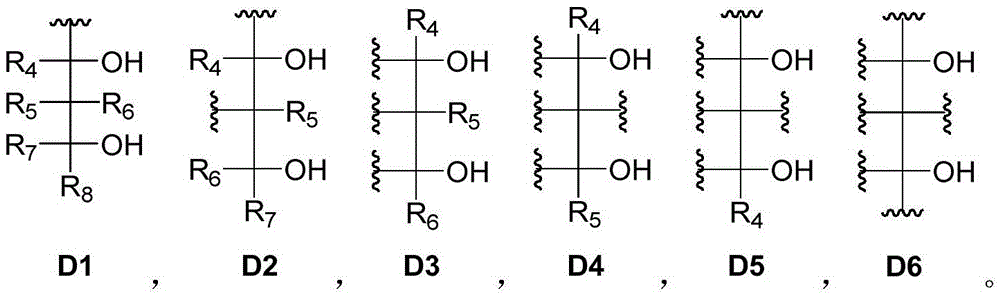
[0179] Component (I) is at least one polyol compound containing three or more diol units, wherein the diol units are selected from 1,2-diol units or 1,3-diol units Alcohol group;
[0180] Component (II) is at least one phenylboronic acid compound containing three or more phenylboronic acid groups, wherein the phenylboronic acid group has an aminomethyl group attached to the ortho-position of the phenylboronic acid;
[0181] Wherein, component (I) contains at least one macromolecular polyol compound, and component (II) contains at least one macromolecular phenylboronic acid compound; wherein, the macromolecular polyol compound and macromolecular phenylboronic acid Compounds whose molecular weight is greater than 1000 Da.
[0182] In the thirteenth embodiment of the present invention, a dynamic polymer material ...
Embodiment 1
[0536] A dynamic polymer material is prepared by using a macromolecular polyol compound containing two diol units and a small molecule phenylboronic acid compound containing three phenylboronic acid units.
[0537]
[0538] Take a certain amount of polyglycerol (a) and dissolve it in methanol solvent to prepare a solution of 0.3mol / L; meanwhile, take a certain amount of phenylboronic acid compound (b) (using diethylenetriamine, 2-formylphenylboronic acid as The raw material, toluene as a solvent, is synthesized by Petasis reaction, wherein the reaction temperature is 90° C., and the reaction time is 10-15 minutes) is dissolved in methanol solvent to prepare a 0.4 mol / L solution. Take 10ml of polyglycerin solution and phenylboronic acid compound solution and add them into a dry and clean beaker. After stirring evenly at room temperature, add a small amount of 1mol / L NaOH solution dropwise and continue stirring. After stirring slowly for about 30-40min, it will have a certain ...
Embodiment 2
[0540] The dynamic polymer material is prepared by using a small molecular polyol compound containing three diol units, a small molecule polyol compound containing three diol units and a small molecule phenylboronic acid compound containing three phenylboronic acid units.
[0541]
[0542] Take a certain amount of polyol compound (a) (using 4,4,4-trihydroxytrimethylbenzene and formaldehyde as raw materials, and synthesize it with zinc nitrate hexahydrate under reflux for 24 hours) and dissolve it in toluene solvent to prepare a 0.2mol / L solution , and take 10ml of the sample and add it to a dry and clean flask, then add 3mg of BHT antioxidant; dissolve 2,3,6,7,10,11-hexahydroxytriphenyl (b) in toluene solvent, configure into a 0.2mol / L solution, and a 10ml sample was taken from it and added to the flask. In the state of stirring, add 0.25mol% phenylboronic acid compound (c) white solid (using 1,3,5-triaminobenzene, 2-formylphenylboronic acid as raw material, toluene as solv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com